Neph1 and nephrin interaction in the slit diaphragm is an important determinant of glomerular permeability
- PMID: 12865409
- PMCID: PMC164293
- DOI: 10.1172/JCI18242
Neph1 and nephrin interaction in the slit diaphragm is an important determinant of glomerular permeability
Abstract
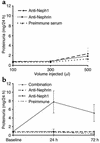
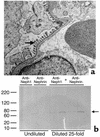
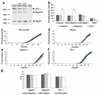
Neph1-deficient mice develop nephrotic syndrome at birth, indicating the importance of this protein in the development of a normal glomerular filtration barrier. While the precise subcellular localization of Neph1 remains unknown, its relationship with other components of the glomerular filtration barrier is of great interest in this field. In this paper, we localize the expression of Neph1 to the glomerular slit diaphragm by immunogold electron microscopy in rodents and describe its direct interaction with two other components of the slit diaphragm, nephrin and ZO-1. Both native and recombinant Neph1 associate with each other as dimers and multimers and interact with nephrin via their extracellular segments. Disruption of the Neph1-nephrin interaction in vivo by injecting combinations of individual subnephritogenic doses of anti-Neph1 and anti-nephrin results in complement- and leukocyte-independent proteinuria with preserved foot processes. This disruption modestly reduces Neph1 and nephrin protein expression in podocytes and dramatically reduces ZO-1 protein expression via the interaction of ZO-1 PDZ domains with the cytoplasmic tail of Neph1, independent of changes in mRNA expression of all three genes. The interaction between nephrin and Neph1 is specific and not shared by either protein with P-cadherin, another integral slit diaphragm protein. The interaction between nephrin and Neph1 therefore appears to be an important determinant of glomerular permeability.
Figures


Similar articles
-
Nephrin and Neph1 co-localize at the podocyte foot process intercellular junction and form cis hetero-oligomers.J Biol Chem. 2003 May 23;278(21):19266-71. doi: 10.1074/jbc.M301279200. Epub 2003 Mar 19. J Biol Chem. 2003. PMID: 12646566
-
Ischemic injury to kidney induces glomerular podocyte effacement and dissociation of slit diaphragm proteins Neph1 and ZO-1.J Biol Chem. 2008 Dec 19;283(51):35579-89. doi: 10.1074/jbc.M805507200. Epub 2008 Oct 14. J Biol Chem. 2008. PMID: 18922801 Free PMC article.
-
Dissociation of NEPH1 from nephrin is involved in development of a rat model of focal segmental glomerulosclerosis.Am J Physiol Renal Physiol. 2008 Nov;295(5):F1376-87. doi: 10.1152/ajprenal.00075.2008. Epub 2008 Aug 20. Am J Physiol Renal Physiol. 2008. PMID: 18715943
-
Molecular basis of the glomerular filtration: nephrin and the emerging protein complex at the podocyte slit diaphragm.Ann Med. 2006;38(7):483-92. doi: 10.1080/07853890600978149. Ann Med. 2006. PMID: 17101539 Review.
-
Unraveling the molecular make-up of the glomerular podocyte slit diaphragm.Exp Nephrol. 2001;9(6):355-9. doi: 10.1159/000052632. Exp Nephrol. 2001. PMID: 11701993 Review.
Cited by
-
Bigenic mouse models of focal segmental glomerulosclerosis involving pairwise interaction of CD2AP, Fyn, and synaptopodin.J Clin Invest. 2006 May;116(5):1337-45. doi: 10.1172/JCI27400. Epub 2006 Apr 20. J Clin Invest. 2006. PMID: 16628251 Free PMC article.
-
Renal cell markers: lighthouses for managing renal diseases.Am J Physiol Renal Physiol. 2021 Dec 1;321(6):F715-F739. doi: 10.1152/ajprenal.00182.2021. Epub 2021 Oct 11. Am J Physiol Renal Physiol. 2021. PMID: 34632812 Free PMC article.
-
Copy-number variation associated with congenital anomalies of the kidney and urinary tract.Pediatr Nephrol. 2015 Mar;30(3):487-95. doi: 10.1007/s00467-014-2962-9. Epub 2014 Oct 1. Pediatr Nephrol. 2015. PMID: 25270717
-
Role of calcineurin (CN) in kidney glomerular podocyte: CN inhibitor ameliorated proteinuria by inhibiting the redistribution of CN at the slit diaphragm.Physiol Rep. 2016 Mar;4(6):e12679. doi: 10.14814/phy2.12679. Physiol Rep. 2016. PMID: 27009276 Free PMC article.
-
Podocyte-secreted angiopoietin-like-4 mediates proteinuria in glucocorticoid-sensitive nephrotic syndrome.Nat Med. 2011 Jan;17(1):117-22. doi: 10.1038/nm.2261. Epub 2010 Dec 12. Nat Med. 2011. PMID: 21151138 Free PMC article.
References
-
- Quaggin SE, et al. The basic-helix-loop-helix protein pod1 is critically important for kidney and lung organogenesis. Development. 1999;126:5771–5783. - PubMed
-
- Orikasa M, Matsui K, Oite T, Shimizu F. Massive proteinuria induced in rats by a single intravenous injection of a monoclonal antibody. J. Immunol. 1988;141:807–814. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

