Activity of Rho-family GTPases during cell division as visualized with FRET-based probes
- PMID: 12860967
- PMCID: PMC2172791
- DOI: 10.1083/jcb.200212049
Activity of Rho-family GTPases during cell division as visualized with FRET-based probes
Abstract
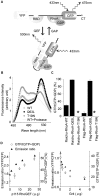
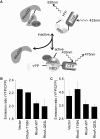
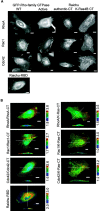
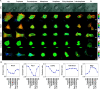
Rho-family GTPases regulate many cellular functions. To visualize the activity of Rho-family GTPases in living cells, we developed fluorescence resonance energy transfer (FRET)-based probes for Rac1 and Cdc42 previously (Itoh, R.E., K. Kurokawa, Y. Ohba, H. Yoshizaki, N. Mochizuki, and M. Matsuda. 2002. Mol. Cell. Biol. 22:6582-6591). Here, we added two types of probes for RhoA. One is to monitor the activity balance between guanine nucleotide exchange factors and GTPase-activating proteins, and another is to monitor the level of GTP-RhoA. Using these FRET probes, we imaged the activities of Rho-family GTPases during the cell division of HeLa cells. The activities of RhoA, Rac1, and Cdc42 were high at the plasma membrane in interphase, and decreased rapidly on entry into M phase. From after anaphase, the RhoA activity increased at the plasma membrane including cleavage furrow. Rac1 activity was suppressed at the spindle midzone and increased at the plasma membrane of polar sides after telophase. Cdc42 activity was suppressed at the plasma membrane and was high at the intracellular membrane compartments during cytokinesis. In conclusion, we could use the FRET-based probes to visualize the complex spatio-temporal regulation of Rho-family GTPases during cell division.
Figures

Similar articles
-
Mechanism and role of localized activation of Rho-family GTPases in growth factor-stimulated fibroblasts and neuronal cells.Biochem Soc Trans. 2005 Aug;33(Pt 4):631-4. doi: 10.1042/BST0330631. Biochem Soc Trans. 2005. PMID: 16042560 Review.
-
FRET imaging in nerve growth cones reveals a high level of RhoA activity within the peripheral domain.Brain Res Mol Brain Res. 2005 Oct 3;139(2):277-87. doi: 10.1016/j.molbrainres.2005.05.030. Brain Res Mol Brain Res. 2005. PMID: 16024133
-
Analysis of the spatiotemporal activation of rho GTPases using Raichu probes.Methods Enzymol. 2006;406:315-32. doi: 10.1016/S0076-6879(06)06023-X. Methods Enzymol. 2006. PMID: 16472667
-
Designing biosensors for Rho family proteins--deciphering the dynamics of Rho family GTPase activation in living cells.J Cell Sci. 2004 Mar 15;117(Pt 8):1313-8. doi: 10.1242/jcs.01117. J Cell Sci. 2004. PMID: 15020671 Review.
-
Rho family GTPases cooperate with p53 deletion to promote primary mouse embryonic fibroblast cell invasion.Oncogene. 2004 Jul 22;23(33):5577-85. doi: 10.1038/sj.onc.1207752. Oncogene. 2004. PMID: 15122327
Cited by
-
Rac1 and Cdc42 GTPases regulate shear stress-driven β-catenin signaling in osteoblasts.Biochem Biophys Res Commun. 2013 Apr 19;433(4):502-7. doi: 10.1016/j.bbrc.2013.03.020. Epub 2013 Mar 21. Biochem Biophys Res Commun. 2013. PMID: 23524265 Free PMC article.
-
MgcRacGAP restricts active RhoA at the cytokinetic furrow and both RhoA and Rac1 at cell-cell junctions in epithelial cells.Mol Biol Cell. 2015 Jul 1;26(13):2439-55. doi: 10.1091/mbc.E14-11-1553. Epub 2015 May 6. Mol Biol Cell. 2015. PMID: 25947135 Free PMC article.
-
Endomembrane control of cell polarity: Relevance to cancer.Small GTPases. 2015;6(2):104-7. doi: 10.1080/21541248.2015.1018402. Small GTPases. 2015. PMID: 26156751 Free PMC article. Review.
-
Patterning of the cell cortex by Rho GTPases.Nat Rev Mol Cell Biol. 2024 Apr;25(4):290-308. doi: 10.1038/s41580-023-00682-z. Epub 2024 Jan 3. Nat Rev Mol Cell Biol. 2024. PMID: 38172611 Review.
-
Microtopographical features generated by photopolymerization recruit RhoA/ROCK through TRPV1 to direct cell and neurite growth.Biomaterials. 2015 Jun;53:95-106. doi: 10.1016/j.biomaterials.2015.02.057. Epub 2015 Mar 12. Biomaterials. 2015. PMID: 25890710 Free PMC article.
References
-
- Castrillon, D.H., and S.A. Wasserman. 1994. Diaphanous is required for cytokinesis in Drosophila and shares domains of similarity with the products of the limb deformity gene. Development. 120:3367–3377. - PubMed
-
- del Pozo, M.A., W.B. Kiosses, N.B. Alderson, N. Meller, K.M. Hahn, and M.A. Schwartz. 2002. Integrins regulate GTP-Rac localized effector interactions through dissociation of Rho-GDI. Nat. Cell Biol. 4:232–239. - PubMed
-
- Drechsel, D.N., A.A. Hyman, A. Hall, and M. Glotzer. 1997. A requirement for Rho and Cdc42 during cytokinesis in Xenopus embryos. Curr. Biol. 7:12–23. - PubMed
-
- Dutartre, H., J. Davoust, J.P. Gorvel, and P. Chavrier. 1996. Cytokinesis arrest and redistribution of actin-cytoskeleton regulatory components in cells expressing the Rho GTPase CDC42Hs. J. Cell Sci. 109:367–377. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

