Microtubule network facilitates nuclear targeting of human cytomegalovirus capsid
- PMID: 12857923
- PMCID: PMC165267
- DOI: 10.1128/jvi.77.15.8541-8547.2003
Microtubule network facilitates nuclear targeting of human cytomegalovirus capsid
Abstract
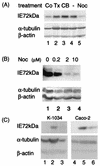
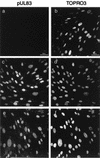
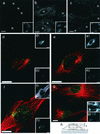
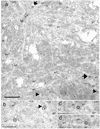
We assessed the requirement of the host cytoskeleton for the intracytosolic transport of incoming human cytomegalovirus (HCMV) capsids. Treatments with microtubule (MT)-depolymerizing drugs nocodazole and colchicine led to a drastic decrease in levels of IE1 antigen, whereas cytochalasin B had no effect on the level of IE1 as determined by Western blot analyses. Sequential treatment including nocodazole washout and removal of cell surface virion revealed that HCMV entry into the cells occurred normally in the absence of the MT network. This finding was also supported by data obtained by monitoring pUL83 signals with an immunofluorescent assay (IFA). Furthermore, we demonstrated a close association of incoming HCMV capsids with MTs by IFA and ultrastructural analyses. In the absence of the MT network, the capsids which had entered the cytoplasm did not move to close proximity of the nucleus. These data suggest that HCMV capsids associate with the MT network to facilitate their own movement to the nucleus before the onset of immediate-early (IE) gene expression and that this association is required to start efficient IE gene expression.
Figures


Similar articles
-
Microtubule-mediated transport of incoming herpes simplex virus 1 capsids to the nucleus.J Cell Biol. 1997 Mar 10;136(5):1007-21. doi: 10.1083/jcb.136.5.1007. J Cell Biol. 1997. PMID: 9060466 Free PMC article.
-
A Role for Myosin Va in Human Cytomegalovirus Nuclear Egress.J Virol. 2018 Feb 26;92(6):e01849-17. doi: 10.1128/JVI.01849-17. Print 2018 Mar 15. J Virol. 2018. PMID: 29298889 Free PMC article.
-
Exploitation of microtubule cytoskeleton and dynein during parvoviral traffic toward the nucleus.J Virol. 2003 Oct;77(19):10270-9. doi: 10.1128/jvi.77.19.10270-10279.2003. J Virol. 2003. PMID: 12970411 Free PMC article.
-
Disruption of PML-associated nuclear bodies by IE1 correlates with efficient early stages of viral gene expression and DNA replication in human cytomegalovirus infection.Virology. 2000 Aug 15;274(1):39-55. doi: 10.1006/viro.2000.0448. Virology. 2000. PMID: 10936087
-
Function of dynein and dynactin in herpes simplex virus capsid transport.Mol Biol Cell. 2002 Aug;13(8):2795-809. doi: 10.1091/mbc.01-07-0348. Mol Biol Cell. 2002. PMID: 12181347 Free PMC article.
Cited by
-
A Novel Entry/Uncoating Assay Reveals the Presence of at Least Two Species of Viral Capsids During Synchronized HIV-1 Infection.PLoS Pathog. 2016 Sep 30;12(9):e1005897. doi: 10.1371/journal.ppat.1005897. eCollection 2016 Sep. PLoS Pathog. 2016. PMID: 27690375 Free PMC article.
-
Human Cytomegalovirus nuclear egress and secondary envelopment are negatively affected in the absence of cellular p53.Virology. 2016 Oct;497:279-293. doi: 10.1016/j.virol.2016.07.021. Epub 2016 Aug 5. Virology. 2016. PMID: 27498410 Free PMC article.
-
Diverse immune evasion strategies by human cytomegalovirus.Immunol Res. 2012 Dec;54(1-3):140-51. doi: 10.1007/s12026-012-8304-8. Immunol Res. 2012. PMID: 22454101 Review.
-
Human cytomegalovirus entry into epithelial and endothelial cells depends on genes UL128 to UL150 and occurs by endocytosis and low-pH fusion.J Virol. 2006 Jan;80(2):710-22. doi: 10.1128/JVI.80.2.710-722.2006. J Virol. 2006. PMID: 16378974 Free PMC article.
-
Sirtuin 2 promotes human cytomegalovirus replication by regulating cell cycle progression.mSystems. 2023 Dec 21;8(6):e0051023. doi: 10.1128/msystems.00510-23. Epub 2023 Nov 2. mSystems. 2023. PMID: 37916830 Free PMC article.
References
-
- Bolovan-Fritts, C., and J. A. Wiedeman. 2001. Human cytomegalovirus strain Toledo lacks a virus-encoded tropism factor required for infection of aortic endothelial cells. J. Infect. Dis. 184:1252-1261. - PubMed
-
- Cavallo, T., K. Graves, N. L. Cole, and T. Albrecht. 1981. Cytomegalovirus: an ultrastructural study of the morphogenesis of nuclear inclusions in human cell culture. J. Gen. Virol. 56:97-104. - PubMed
-
- Chen, D. H., H. Jiang, M. Lee, F. Liu, and Z. H. Zhou. 1999. Three-dimensional visualization of tegument/capsid interactions in the intact human cytomegalovirus. Virology 260:10-16. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

