Cholesterol depletion of human immunodeficiency virus type 1 and simian immunodeficiency virus with beta-cyclodextrin inactivates and permeabilizes the virions: evidence for virion-associated lipid rafts
- PMID: 12857892
- PMCID: PMC165256
- DOI: 10.1128/jvi.77.15.8237-8248.2003
Cholesterol depletion of human immunodeficiency virus type 1 and simian immunodeficiency virus with beta-cyclodextrin inactivates and permeabilizes the virions: evidence for virion-associated lipid rafts
Abstract
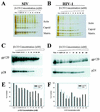
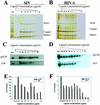
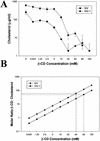
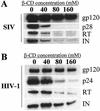
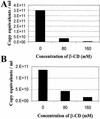
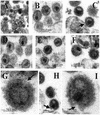
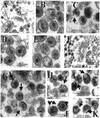
Recent evidence suggests that human immunodeficiency virus type 1 (HIV-1) particles assemble and bud selectively through areas in the plasma membrane of cells that are highly enriched with glycosylphosphatidylinositol-anchored proteins and cholesterol, called lipid rafts. Since cholesterol is required to maintain lipid raft structure and function, we proposed that virion-associated cholesterol removal with the compound 2-hydroxy-propyl-beta-cyclodextrin (beta-CD) might be disruptive to HIV-1 and simian immunodeficiency virus (SIV). We examined the effect of beta-CD on the structure and infectivity of cell-free virions. We found that beta-CD inactivated HIV-1 and SIV in a dose-dependent manner and permeabilized the viral membranes, resulting in the loss of mature Gag proteins (capsid, matrix, nucleocapsid, p1, and p6) without loss of the envelope glycoproteins. SIV also lost reverse transcriptase (RT), integrase (IN), and viral RNA. IN appeared to be only slightly diminished in HIV-1, and viral RNA, RT, matrix, and nucleocapsid proteins were retained in HIV-1 but to a much lesser degree. Host proteins located internally in the virus (actin, moesin, and ezrin) and membrane-associated host proteins (major histocompatibility complex classes I and II) remained associated with the treated virions. Electron microscopy revealed that under conditions that permeabilized the viruses, holes were present in the viral membranes and the viral core structure was perturbed. These data provide evidence that an intact viral membrane is required to maintain mature virion core integrity. Since the viruses were not fixed before beta-CD treatment and intact virion particles were recovered, the data suggest that virions may possess a protein scaffold that can maintain overall structure despite disruptions in membrane integrity.
Figures


Similar articles
-
Lipid rafts and HIV pathogenesis: virion-associated cholesterol is required for fusion and infection of susceptible cells.AIDS Res Hum Retroviruses. 2003 Aug;19(8):675-87. doi: 10.1089/088922203322280900. AIDS Res Hum Retroviruses. 2003. PMID: 13678470
-
Cholesterol depletion inactivates XMRV and leads to viral envelope protein release from virions: evidence for role of cholesterol in XMRV infection.PLoS One. 2012;7(10):e48013. doi: 10.1371/journal.pone.0048013. Epub 2012 Oct 26. PLoS One. 2012. PMID: 23110160 Free PMC article.
-
Vaccinia virus penetration requires cholesterol and results in specific viral envelope proteins associated with lipid rafts.J Virol. 2005 Feb;79(3):1623-34. doi: 10.1128/JVI.79.3.1623-1634.2005. J Virol. 2005. PMID: 15650188 Free PMC article.
-
Sites, mechanism of action and lack of reversibility of primate lentivirus inactivation by preferential covalent modification of virion internal proteins.Curr Mol Med. 2003 May;3(3):265-72. doi: 10.2174/1566524033479889. Curr Mol Med. 2003. PMID: 12699362 Review.
-
Exploring HIV-1 Maturation: A New Frontier in Antiviral Development.Viruses. 2024 Sep 6;16(9):1423. doi: 10.3390/v16091423. Viruses. 2024. PMID: 39339899 Free PMC article. Review.
Cited by
-
Trimer Enhancement Mutation Effects on HIV-1 Matrix Protein Binding Activities.J Virol. 2016 May 27;90(12):5657-5664. doi: 10.1128/JVI.00509-16. Print 2016 Jun 15. J Virol. 2016. PMID: 27030269 Free PMC article.
-
Dose-Response Effects of 7-Dehydrocholesterol Reductase Inhibitors on Sterol Profiles and Vesicular Stomatitis Virus Replication.ACS Pharmacol Transl Sci. 2022 Oct 25;5(11):1086-1096. doi: 10.1021/acsptsci.2c00051. eCollection 2022 Nov 11. ACS Pharmacol Transl Sci. 2022. PMID: 36407960 Free PMC article.
-
Retinoic acid and liver X receptor agonist synergistically inhibit HIV infection in CD4+ T cells by up-regulating ABCA1-mediated cholesterol efflux.Lipids Health Dis. 2012 Jul 9;11:69. doi: 10.1186/1476-511X-11-69. Lipids Health Dis. 2012. PMID: 22676378 Free PMC article.
-
Line tension at lipid phase boundaries as driving force for HIV fusion peptide-mediated fusion.Nat Commun. 2016 Apr 26;7:11401. doi: 10.1038/ncomms11401. Nat Commun. 2016. PMID: 27113279 Free PMC article.
-
The Role of Lipids in Retrovirus Replication.Viruses. 2010 May 1;2(5):1146-1180. doi: 10.3390/v2051146. Viruses. 2010. PMID: 20740061 Free PMC article.
References
-
- Arthur, L. O., J. W. Bess, Jr., E. N. Chertova, J. L. Rossio, M. T. Esser, R. E. Benveniste, L. E. Henderson, and J. D. Lifson. 1998. Chemical inactivation of retroviral infectivity by targeting nucleocapsid protein zinc fingers: a candidate SIV vaccine. AIDS Res. Hum. Retrovir. 14:S311-S319. - PubMed
-
- Benveniste, R. E., D. Raben, R. W. Hill, W. B. Knott, J. E. Drummond, L. O. Arthur, P. B. Jahrling, W. R. Morton, L. E. Henderson, and G. Heidecker. 1989. Molecular characterization and comparison of simian immunodeficiency virus isolates from macaques, mangabeys, and African green monkeys. J. Med. Primatol. 18:287-303. - PubMed
-
- Bess, J. W., Jr., R. J. Gorelick, W. J. Bosche, L. E. Henderson, and L. O. Arthur. 1997. Microvesicles are a source of contaminating cellular proteins found in purified HIV-1 preparations. Virology 230:134-144. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

