HIV-1 Nef intersects the macrophage CD40L signalling pathway to promote resting-cell infection
- PMID: 12853962
- PMCID: PMC9524218
- DOI: 10.1038/nature01749
HIV-1 Nef intersects the macrophage CD40L signalling pathway to promote resting-cell infection
Abstract
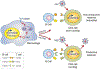
All primate lentiviruses (HIV-1, HIV-2, SIV) encode Nef proteins, which are important for viral replication and pathogenicity in vivo. It is not known how Nef regulates these processes. It has been suggested that Nef protects infected cells from apoptosis and recognition by cytotoxic T lymphocytes. Other studies suggest that Nef influences the activation state of the infected cell, thereby enhancing the ability of that cell to support viral replication. Here we show that macrophages that express Nef or are stimulated through the CD40 receptor release a paracrine factor that renders T lymphocytes permissive to HIV-1 infection. This activity requires the upregulation of B-cell receptors involved in the alternative pathway of T-lymphocyte stimulation. T lymphocytes stimulated through this pathway become susceptible to viral infection without progressing through the cell cycle. We identify two proteins, soluble CD23 and soluble ICAM, that are induced from macrophages by Nef and CD40L, and which mediate their effects on lymphocyte permissivity. Our results reveal a mechanism by which Nef expands the cellular reservoir of HIV-1 by permitting the infection of resting T lymphocytes.
Figures



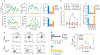
Comment in
-
HIV: cross-talk and viral reservoirs.Nature. 2003 Jul 10;424(6945):136-7. doi: 10.1038/424136a. Nature. 2003. PMID: 12853937 No abstract available.
Similar articles
-
HIV: cross-talk and viral reservoirs.Nature. 2003 Jul 10;424(6945):136-7. doi: 10.1038/424136a. Nature. 2003. PMID: 12853937 No abstract available.
-
HIV-1 Nef mediates lymphocyte chemotaxis and activation by infected macrophages.Nat Med. 1999 Sep;5(9):997-103. doi: 10.1038/12433. Nat Med. 1999. PMID: 10470075 Free PMC article.
-
Resistance to apoptosis in HIV-infected CD4+ T lymphocytes is mediated by macrophages: role for Nef and immune activation in viral persistence.J Immunol. 2000 Dec 1;165(11):6437-46. doi: 10.4049/jimmunol.165.11.6437. J Immunol. 2000. PMID: 11086083
-
HIV1 Nef: the Machiavelli of cellular activation.Res Virol. 1997 Jan-Feb;148(1):58-64. doi: 10.1016/s0923-2516(97)81915-2. Res Virol. 1997. PMID: 9017836 Review. No abstract available.
-
Nef: a pleiotropic modulator of primate lentivirus infectivity and pathogenesis.Acta Microbiol Immunol Hung. 2006 Mar;53(1):51-75. doi: 10.1556/AMicr.53.2006.1.4. Acta Microbiol Immunol Hung. 2006. PMID: 16696550 Review.
Cited by
-
HIV accessory proteins and surviving the host cell.Curr HIV/AIDS Rep. 2004 Apr;1(1):47-53. doi: 10.1007/s11904-004-0007-x. Curr HIV/AIDS Rep. 2004. PMID: 16091223 Review.
-
Anti-CD45RO suppresses human immunodeficiency virus type 1 replication in microglia: role of Hck tyrosine kinase and implications for AIDS dementia.J Virol. 2006 Jan;80(1):62-72. doi: 10.1128/JVI.80.1.62-72.2006. J Virol. 2006. PMID: 16352531 Free PMC article.
-
Kinetics of human immunodeficiency virus type 1 decay following entry into resting CD4+ T cells.J Virol. 2005 Feb;79(4):2199-210. doi: 10.1128/JVI.79.4.2199-2210.2005. J Virol. 2005. PMID: 15681422 Free PMC article.
-
HIV-1 Nef induces proinflammatory state in macrophages through its acidic cluster domain: involvement of TNF alpha receptor associated factor 2.PLoS One. 2011;6(8):e22982. doi: 10.1371/journal.pone.0022982. Epub 2011 Aug 23. PLoS One. 2011. PMID: 21886773 Free PMC article.
-
HIV type 1 infection up-regulates TLR2 and TLR4 expression and function in vivo and in vitro.AIDS Res Hum Retroviruses. 2012 Oct;28(10):1313-28. doi: 10.1089/aid.2011.0297. Epub 2012 Mar 12. AIDS Res Hum Retroviruses. 2012. PMID: 22280204 Free PMC article.
References
-
- Kirchhoff F, Greenough TC, Brettler DB, Sullivan JL & Desrosiers RC Brief report: absence of intact nef sequences in a long-term survivor with nonprogressive HIV-1 infection. N. Engl. J. Med 332, 228–232 (1995). - PubMed
-
- Deacon NJ et al. Genomic structure of an attenuated quasi species of HIV-1 from a blood transfusion donor and recipients. Science 270, 988–991 (1995). - PubMed
-
- Kestler HW et al. Importance of the nef gene for maintenance of high virus loads and for development of AIDS. Cell 65, 651–662 (1991). - PubMed
-
- Johnson WE & Desrosiers RC Viral persistence: HIV’s strategies of immune system evasion. Annu. Rev. Med 53, 499–518 (2002). - PubMed
-
- Collins KL & Baltimore D HIV’s evasion of the cellular immune response. Immunol. Rev 168, 65–74 (1999). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

