Epithelial Na, K-ATPase expression is down-regulated in canine prostate cancer; a possible consequence of metabolic transformation in the process of prostate malignancy
- PMID: 12848899
- PMCID: PMC194866
- DOI: 10.1186/1475-2867-3-8
Epithelial Na, K-ATPase expression is down-regulated in canine prostate cancer; a possible consequence of metabolic transformation in the process of prostate malignancy
Abstract
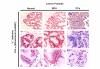
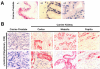
BACKGROUND: An important physiological function of the normal prostate gland is the synthesis and secretion of a citrate rich prostatic fluid. In prostate cancer, citrate production levels are reduced as a result of altered cellular metabolism and bioenergetics. Na, K-ATPase is essential for citrate production since the inward Na+ gradients it generates are utilized for the Na+ dependent uptake of aspartate, a major substrate for citrate synthesis. The objective of this study was to compare the expression of previously identified Na, K-ATPase isoforms in normal canine prostate, benign prostatic hyperplasia (BPH) and prostatic adenocarcinoma (PCa) using immunohistochemistry in order to determine whether reduced citrate levels in PCa are also accompanied by changes in Na, K-ATPase expression. RESULTS: Expression of Na, K-ATPase alpha1 and beta1 isoforms was observed in the lateral and basolateral plasma membrane domains of prostatic epithelial cells in normal and BPH prostates. Canine kidney was used as positive control for expression of Na, K-ATPase alpha1 and gamma isoforms. The alpha1 isoform was detected in abundance in prostatic epithelial cells but there was no evidence of alpha2, alpha3 or gamma subunit expression. In advanced PCa, Na, K-ATPase alpha1 isoform expression was significantly lower compared to normal and BPH glands. The abundant basolateral immunostaining observed in normal and BPH tissue was significantly attenuated in PCa. CONCLUSION: The loss of epithelial structure and function and the transformation of normal epithelial cells to malignant cells in the canine prostate have important implications for cellular metabolism and are accompanied by a down regulation of Na, K-ATPase.
Figures



Similar articles
-
Isoforms of Na+, K+-ATPase in human prostate; specificity of expression and apical membrane polarization.Histol Histopathol. 2001 Jan;16(1):141-54. doi: 10.14670/HH-16.141. Histol Histopathol. 2001. PMID: 11193188
-
Expression and cellular localization of Na,K-ATPase isoforms in the rat ventral prostate.BJU Int. 2003 Nov;92(7):793-802. doi: 10.1046/j.1464-410x.2003.04460.x. BJU Int. 2003. PMID: 14616469
-
Na+/K+ ATPase α1 and β3 subunits are localized to the basolateral membrane of trophectoderm cells in human blastocysts.Hum Reprod. 2022 Jun 30;37(7):1423-1430. doi: 10.1093/humrep/deac124. Hum Reprod. 2022. PMID: 35640043 Free PMC article.
-
The cardiac sodium pump: structure and function.Basic Res Cardiol. 2002;97 Suppl 1:I19-24. doi: 10.1007/s003950200024. Basic Res Cardiol. 2002. PMID: 12479229 Review.
-
Neuronal function and alpha3 isoform of the Na/K-ATPase.Front Biosci. 2005 Sep 1;10:2373-96. doi: 10.2741/1704. Front Biosci. 2005. PMID: 15970502 Review.
Cited by
-
Image analysis tools for evaluation of microscopic views of immunohistochemically stained specimen in medical research-a review.J Med Syst. 2012 Aug;36(4):2621-31. doi: 10.1007/s10916-011-9737-7. Epub 2011 May 17. J Med Syst. 2012. PMID: 21584771 Review.
-
"The Lower Threshold" phenomenon in tumor cells toward endogenous digitalis-like compounds: Responsible for tumorigenesis?J Carcinog. 2012;11:2. doi: 10.4103/1477-3163.92999. Epub 2012 Feb 17. J Carcinog. 2012. PMID: 22438768 Free PMC article.
-
A Direct Comparison of the Anticancer Activities of Digitoxin MeON-Neoglycosides and O-Glycosides: Oligosaccharide Chain Length-Dependent Induction of Caspase-9-Mediated Apoptosis.ACS Med Chem Lett. 2010 Jul 12;1(7):326-330. doi: 10.1021/ml1000933. ACS Med Chem Lett. 2010. PMID: 21103068 Free PMC article.
-
Na,K-ATPase Isozymes in Colorectal Cancer and Liver Metastases.Front Physiol. 2016 Jan 29;7:9. doi: 10.3389/fphys.2016.00009. eCollection 2016. Front Physiol. 2016. PMID: 26858653 Free PMC article.
-
Downregulation of ATP1A1 promotes cancer development in renal cell carcinoma.Clin Proteomics. 2017 May 4;14:15. doi: 10.1186/s12014-017-9150-4. eCollection 2017. Clin Proteomics. 2017. PMID: 28484360 Free PMC article.
References
-
- Kavanagh JP. Sodium, potassium, calcium, magnesium, zinc, citrate and chloride content of human prostatic and seminal fluid. J Reprod Fertil. 1985;75:35–41. - PubMed
-
- Franklin RB, Lao LX, Costello LC. Evidence for two aspartate transport systems in prostate epithelial cells. Prostate. 1990;16:137–45. - PubMed
-
- Smith ER. The secretion of electrolytes by the pilocarpine-stimulated canine prostate gland. Proc Soc Exp Biol Med. 1969;132:223–6. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

