Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) infection of human fibroblast cells occurs through endocytosis
- PMID: 12829837
- PMCID: PMC161913
- DOI: 10.1128/jvi.77.14.7978-7990.2003
Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) infection of human fibroblast cells occurs through endocytosis
Abstract
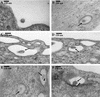
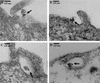
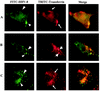
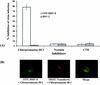
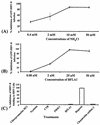
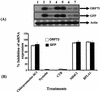
Kaposi's sarcoma (KS)-associated herpesvirus or human herpesvirus 8 (HHV-8) DNA and transcripts have been detected in the B cells, macrophages, keratinocytes, and endothelial and epithelial cells of KS patients. In vitro, HHV-8 infects human B, endothelial, epithelial, and fibroblast cells, as well as animal cells, and the infection is characterized by (i) absence of lytic replication by the input virus and (ii) latent infection. For its initial binding to target cells, HHV-8 uses ubiquitous heparan sulfate molecules via its envelope-associated glycoproteins gB and gpK8.1A. HHV-8 also interacts with the alpha3beta1 integrin via its glycoprotein gB, and virus binding studies suggest that alpha3beta1 is one of the HHV-8 entry receptors (S. M. Akula, N. P. Pramod, F. Z. Wang, and B. Chandran, Cell 108:407-419, 2002). In this study, morphological and biochemical techniques were used to examine the entry of HHV-8 into human foreskin fibroblasts (HFF). HHV-8 was detected in coated vesicles and in large, smooth-surfaced endocytic vesicles. Fusion of viral envelope with the vesicle wall was also observed. In immune electron microscopy, anti-HHV-8 gB antibodies colocalized with virus-containing endocytic vesicles. In fluorescence microscopic analyses, transferrin was colocalized with HHV-8. HHV-8 infection was significantly inhibited by preincubation of cells with chlorpromazine HCl, which blocks endocytosis via clathrin-coated pits, but not by nystatin and cholera toxin B, which blocks endocytosis via caveolae and induces the dissociation of lipid rafts, respectively. Infection was also inhibited by blocking the acidification of endosomes by NH(4)Cl and bafilomycin A. Inhibition of HHV-8 open reading frame 73 gene expression by chlorpromazine HCl, bafilomycin A, and NH(4)Cl demonstrated that the virions in the vesicles could proceed to cause an infection. Taken together, these findings suggest that for its infectious entry into HFF, HHV-8 uses clathrin-mediated endocytosis and a low-pH intracellular environment.
Figures
Similar articles
-
Kaposi's sarcoma-associated herpesvirus utilizes an actin polymerization-dependent macropinocytic pathway to enter human dermal microvascular endothelial and human umbilical vein endothelial cells.J Virol. 2009 May;83(10):4895-911. doi: 10.1128/JVI.02498-08. Epub 2009 Mar 11. J Virol. 2009. PMID: 19279100 Free PMC article.
-
Human herpesvirus 8 envelope glycoprotein B mediates cell adhesion via its RGD sequence.J Virol. 2003 Mar;77(5):3131-47. doi: 10.1128/jvi.77.5.3131-3147.2003. J Virol. 2003. PMID: 12584338 Free PMC article.
-
Host gene induction and transcriptional reprogramming in Kaposi's sarcoma-associated herpesvirus (KSHV/HHV-8)-infected endothelial, fibroblast, and B cells: insights into modulation events early during infection.Cancer Res. 2004 Jan 1;64(1):72-84. doi: 10.1158/0008-5472.can-03-2767. Cancer Res. 2004. PMID: 14729610
-
Kaposi's Sarcoma (KS)-associated herpesvirus and its role in KS.Infect Agents Dis. 1996 Oct;5(4):215-22. Infect Agents Dis. 1996. PMID: 8884366 Review.
-
The role of HHV-8 in Kaposi's sarcoma.Semin Cancer Biol. 1999 Jun;9(3):151-64. doi: 10.1006/scbi.1999.0129. Semin Cancer Biol. 1999. PMID: 10343067 Review.
Cited by
-
Kaposi sarcoma-associated herpesvirus (KSHV) induces a functional tumor-associated phenotype for oral fibroblasts.Cancer Lett. 2012 May 28;318(2):214-20. doi: 10.1016/j.canlet.2011.12.019. Epub 2011 Dec 17. Cancer Lett. 2012. PMID: 22186301 Free PMC article.
-
Focal adhesion kinase is critical for entry of Kaposi's sarcoma-associated herpesvirus into target cells.J Virol. 2006 Feb;80(3):1167-80. doi: 10.1128/JVI.80.3.1167-1180.2006. J Virol. 2006. PMID: 16414994 Free PMC article.
-
The m74 gene product of murine cytomegalovirus (MCMV) is a functional homolog of human CMV gO and determines the entry pathway of MCMV.J Virol. 2010 May;84(9):4469-80. doi: 10.1128/JVI.02441-09. Epub 2010 Feb 24. J Virol. 2010. PMID: 20181688 Free PMC article.
-
Localization of human herpesvirus type 8 in human sperms by in situ PCR.J Mol Histol. 2005 Sep;36(6-7):401-12. doi: 10.1007/s10735-005-9010-9. Epub 2006 Jan 10. J Mol Histol. 2005. PMID: 16402152
-
[Humanes herpesvirus 8 (HHV-8) and Kaposi sarcoma].Hautarzt. 2008 Jan;59(1):18-25. doi: 10.1007/s00105-007-1445-3. Hautarzt. 2008. PMID: 18209996 Review. German.
References
-
- Akula, S. M., N. P. Pramod, F. Z. Wang, and B. Chandran. 2001. Human herpesvirus envelope-associated glycoprotein B interacts with heparan sulfate-like moieties. Virology 284:235-249. - PubMed
-
- Akula, S. M., F. Z. Wang, J. Vieira, and B. Chandran. 2001. Human herpesvirus 8 (HHV-8/KSHV) infection of target cells involves interaction with heparan sulfate. Virology 282:245-255. - PubMed
-
- Akula, S. M., D. J. Hurley, R. L. Wixon, C. Wang, and C. C. Chase. 2002. Effect of genistein on replication of bovine herpesvirus type 1. Am. J. Vet. Res. 63:1124-1128. - PubMed
-
- Akula, S. M., N. P. Pramod, F. Z. Wang, and B. Chandran. 2002. Integrin α3β1 (CD 49c/29) is a cellular receptor for Kaposi's sarcoma-associated herpesvirus (KSHV/HHV-8) entry into the target cells. Cell 108:407-419. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

