The Ebola virus VP35 protein inhibits activation of interferon regulatory factor 3
- PMID: 12829834
- PMCID: PMC161945
- DOI: 10.1128/jvi.77.14.7945-7956.2003
The Ebola virus VP35 protein inhibits activation of interferon regulatory factor 3
Abstract
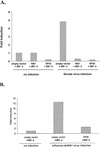
The Ebola virus VP35 protein was previously found to act as an interferon (IFN) antagonist which could complement growth of influenza delNS1 virus, a mutant influenza virus lacking the influenza virus IFN antagonist protein, NS1. The Ebola virus VP35 could also prevent the virus- or double-stranded RNA-mediated transcriptional activation of both the beta IFN (IFN-beta) promoter and the IFN-stimulated ISG54 promoter (C. Basler et al., Proc. Natl. Acad. Sci. USA 97:12289-12294, 2000). We now show that VP35 inhibits virus infection-induced transcriptional activation of IFN regulatory factor 3 (IRF-3)-responsive mammalian promoters and that VP35 does not block signaling from the IFN-alpha/beta receptor. The ability of VP35 to inhibit this virus-induced transcription correlates with its ability to block activation of IRF-3, a cellular transcription factor of central importance in initiating the host cell IFN response. We demonstrate that VP35 blocks the Sendai virus-induced activation of two promoters which can be directly activated by IRF-3, namely, the ISG54 promoter and the ISG56 promoter. Further, expression of VP35 prevents the IRF-3-dependent activation of the IFN-alpha4 promoter in response to viral infection. The inhibition of IRF-3 appears to occur through an inhibition of IRF-3 phosphorylation. VP35 blocks virus-induced IRF-3 phosphorylation and subsequent IRF-3 dimerization and nuclear translocation. Consistent with these observations, Ebola virus infection of Vero cells activated neither transcription from the ISG54 promoter nor nuclear accumulation of IRF-3. These data suggest that in Ebola virus-infected cells, VP35 inhibits the induction of antiviral genes, including the IFN-beta gene, by blocking IRF-3 activation.
Figures





Similar articles
-
Ebola virus VP35 protein binds double-stranded RNA and inhibits alpha/beta interferon production induced by RIG-I signaling.J Virol. 2006 Jun;80(11):5168-78. doi: 10.1128/JVI.02199-05. J Virol. 2006. PMID: 16698997 Free PMC article.
-
The VP35 protein of Ebola virus inhibits the antiviral effect mediated by double-stranded RNA-dependent protein kinase PKR.J Virol. 2007 Jan;81(1):182-92. doi: 10.1128/JVI.01006-06. Epub 2006 Oct 25. J Virol. 2007. PMID: 17065211 Free PMC article.
-
Reverse genetic generation of recombinant Zaire Ebola viruses containing disrupted IRF-3 inhibitory domains results in attenuated virus growth in vitro and higher levels of IRF-3 activation without inhibiting viral transcription or replication.J Virol. 2006 Jul;80(13):6430-40. doi: 10.1128/JVI.00044-06. J Virol. 2006. PMID: 16775331 Free PMC article.
-
Evasion of interferon responses by Ebola and Marburg viruses.J Interferon Cytokine Res. 2009 Sep;29(9):511-20. doi: 10.1089/jir.2009.0076. J Interferon Cytokine Res. 2009. PMID: 19694547 Free PMC article. Review.
-
On the role of IRF in host defense.J Interferon Cytokine Res. 2002 Jan;22(1):59-71. doi: 10.1089/107999002753452665. J Interferon Cytokine Res. 2002. PMID: 11846976 Review.
Cited by
-
Foot-and-mouth disease virus 3C protease cleaves NEMO to impair innate immune signaling.J Virol. 2012 Sep;86(17):9311-22. doi: 10.1128/JVI.00722-12. Epub 2012 Jun 20. J Virol. 2012. PMID: 22718831 Free PMC article.
-
Hemorrhagic fever viruses: Pathogenesis, therapeutics, and emerging and re-emerging potential.Front Microbiol. 2022 Oct 25;13:1040093. doi: 10.3389/fmicb.2022.1040093. eCollection 2022. Front Microbiol. 2022. PMID: 36386719 Free PMC article. Review.
-
Actin cytoskeleton remodeling primes RIG-I-like receptor activation.Cell. 2022 Sep 15;185(19):3588-3602.e21. doi: 10.1016/j.cell.2022.08.011. Cell. 2022. PMID: 36113429 Free PMC article.
-
Mutual antagonism between the Ebola virus VP35 protein and the RIG-I activator PACT determines infection outcome.Cell Host Microbe. 2013 Jul 17;14(1):74-84. doi: 10.1016/j.chom.2013.06.010. Cell Host Microbe. 2013. PMID: 23870315 Free PMC article.
-
Marburg virus VP35 can both fully coat the backbone and cap the ends of dsRNA for interferon antagonism.PLoS Pathog. 2012 Sep;8(9):e1002916. doi: 10.1371/journal.ppat.1002916. Epub 2012 Sep 13. PLoS Pathog. 2012. PMID: 23028316 Free PMC article.
References
-
- Andrejeva, J., D. F. Young, S. Goodbourn, and R. E. Randall. 2002. Degradation of STAT1 and STAT2 by the V proteins of simian virus 5 and human parainfluenza virus type 2, respectively: consequences for virus replication in the presence of alpha/beta and gamma interferons. J. Virol. 76:2159-2167. - PMC - PubMed
-
- Barnes, B., B. Lubyova, and P. M. Pitha. 2002. On the role of IRF in host defense. J. Interferon Cytokine Res. 22:59-71. - PubMed
-
- Barnes, B. J., P. A. Moore, and P. M. Pitha. 2001. Virus-specific activation of a novel interferon regulatory factor, IRF-5, results in the induction of distinct interferon alpha genes. J. Biol. Chem. 276:23382-23390. - PubMed
-
- Basler, C. F., and A. Garcia-Sastre. 2002. Viruses and the type I interferon antiviral system: induction and evasion. Int. Rev. Immunol., in press. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

