Human blood-derived macrophages enhance barrier function of cultured primary bovine and human brain capillary endothelial cells
- PMID: 12829721
- PMCID: PMC2343297
- DOI: 10.1113/jphysiol.2003.045880
Human blood-derived macrophages enhance barrier function of cultured primary bovine and human brain capillary endothelial cells
Abstract
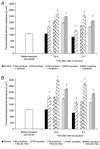
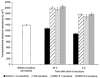
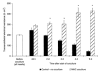
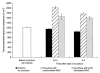
The characteristic properties of the blood-brain barrier (BBB) forming brain capillary endothelial cells (BCEC) are modulated by their microenvironment, but the cellular sources of the induction signals are still unclear. Apart from astrocytes, another cell type in close contact with cerebral blood vessels is the perivascular macrophages, which are known to be regularly replaced by blood-derived monocytic precursor cells. It is unknown if, and how, these cells may interact with the cerebral endothelium and modulate its BBB-specific functions. In the present study, a cell culture model of the BBB was used to investigate the effect of blood-derived human macrophages on the permeability of cultured bovine and human BCEC, determined by a transendothelial electrical resistance (TEER) measurement. We found that the TEER of postconfluent BCEC was considerably increased by a non-contact coculture with macrophages. After 24 h, we found a TEER augmentation of over 50% compared with the control without coculture, and this effect was comparable to the response of BCEC to a C6 glioma cells coculture. Stimulation or HIV-1 infection of the macrophages did not alter their effect on BCEC monolayer permeability. Investigation of signal transduction pathways showed that TEER increase of BCEC due to macrophage coculture was cAMP-independent and involves neither phospholipase C, protein kinase C nor calmodulin. Our findings demonstrate that macrophages are able to modulate BBB-specific functions in cultured BCEC. Thus, these cells or cerebral cells of monocytic origin (e.g. perivascular macrophages), may be part of the microenvironment of BCEC that modulates their specific properties in vivo.
Figures

Similar articles
-
Induction of blood-brain barrier properties in cultured brain capillary endothelial cells: comparison between primary glial cells and C6 cell line.Glia. 2005 Aug 15;51(3):187-98. doi: 10.1002/glia.20189. Glia. 2005. PMID: 15800928
-
Influence of basolateral condition on the regulation of brain microvascular endothelial tight junction properties and barrier function.Brain Res. 2008 Feb 8;1193:84-92. doi: 10.1016/j.brainres.2007.11.072. Epub 2007 Dec 14. Brain Res. 2008. PMID: 18177846
-
Development of an in vitro blood-brain barrier model to study molecular neuropathogenesis and neurovirologic disorders induced by human immunodeficiency virus type 1 infection.J Hum Virol. 2000 Nov-Dec;3(6):324-34. J Hum Virol. 2000. PMID: 11100913
-
Brain endothelial cells and the glio-vascular complex.Cell Tissue Res. 2009 Jan;335(1):75-96. doi: 10.1007/s00441-008-0658-9. Epub 2008 Jul 16. Cell Tissue Res. 2009. PMID: 18633647 Review.
-
The critical component to establish in vitro BBB model: Pericyte.Brain Res Brain Res Rev. 2005 Dec 15;50(2):258-65. doi: 10.1016/j.brainresrev.2005.07.004. Epub 2005 Sep 30. Brain Res Brain Res Rev. 2005. PMID: 16199092 Review.
Cited by
-
The blood-brain barrier in Alzheimer's disease.Neurobiol Dis. 2017 Nov;107:41-56. doi: 10.1016/j.nbd.2016.07.007. Epub 2016 Jul 15. Neurobiol Dis. 2017. PMID: 27425887 Free PMC article. Review.
-
Absence of colony stimulation factor-1 receptor results in loss of microglia, disrupted brain development and olfactory deficits.PLoS One. 2011;6(10):e26317. doi: 10.1371/journal.pone.0026317. Epub 2011 Oct 27. PLoS One. 2011. PMID: 22046273 Free PMC article.
-
The Expanding Cell Diversity of the Brain Vasculature.Front Physiol. 2020 Dec 3;11:600767. doi: 10.3389/fphys.2020.600767. eCollection 2020. Front Physiol. 2020. PMID: 33343397 Free PMC article. Review.
-
Methodologies to assess drug permeation through the blood-brain barrier for pharmaceutical research.Pharm Res. 2013 Nov;30(11):2729-56. doi: 10.1007/s11095-013-1119-z. Epub 2013 Jun 26. Pharm Res. 2013. PMID: 23801086 Review.
-
Perivascular macrophages in health and disease.Nat Rev Immunol. 2018 Nov;18(11):689-702. doi: 10.1038/s41577-018-0056-9. Nat Rev Immunol. 2018. PMID: 30127389 Review.
References
-
- Achim CL, Wiley CA. Inflammation in AIDS and the role of the macrophage in brain pathology. Curr Opin Neurol. 1996;9:221–225. - PubMed
-
- Allt G, Lawrenson JG. Is the pial microvessel a good model for blood-brain barrier studies. Brain Res Brain Res Rev. 1997;24:67–76. - PubMed
-
- Andreesen R, Picht J, Löhr GW. Primary cultures of human blood-born macrophages grown on hydrophobic Teflon membrances. J Immunol Methods. 1983;56:295–304. - PubMed
-
- Bechmann I, Priller J, Kovac A, Bontert M, Wehner T, Klett FF, Bohsung J, Stuschke M, Dirnagl U, Nitsch R. Immune surveillance of mouse brain perivascular spaces by blood-borne macrophages. Eur J Neurosci. 2001;14:1651–1658. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

