Control of the oxidative burst of human neutrophils by staphylococcal leukotoxins
- PMID: 12819053
- PMCID: PMC161991
- DOI: 10.1128/IAI.71.7.3724-3729.2003
Control of the oxidative burst of human neutrophils by staphylococcal leukotoxins
Abstract
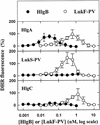
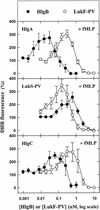
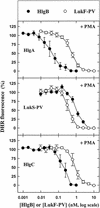
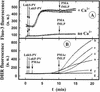
The ability of staphylococcal two-component leukotoxins to induce an oxidative burst and/or to prime human polymorphonuclear cells (PMNs) was studied by using spectrofluorometry or flow cytometry. At sublytic concentrations, the HlgA-HlgB, HlgA-LukF-PV, LukS-PV-LukF-PV, and HlgC-LukF-PV combinations of leukotoxins, but not the LukS-PV-HlgB and HlgC-HlgB combinations, were able to induce H(2)O(2) production similar to the H(2)O(2) production induced by 1 micro M N-formyl-Met-Leu-Phe (fMLP). In addition, when added at sublytic concentrations, all of the leukotoxin combinations primed PMNs for H(2)O(2) production induced by fMLP. Leukotoxin activation was dependent on the presence of Ca(2+) and was inhibited by wortmannin, an inhibitor of phosphatidylinositol 3-kinase, but not by N-methyl-L-arginine, an inhibitor of NO generation, which eliminates the possibility that NO plays a role in the action of leukotoxins. At higher concentrations, all leukotoxins inhibited H(2)O(2) production by PMNs activated by fMLP, phorbol 12-myristate 13-acetate (PMA), or the leukotoxins themselves. This inhibition was not related to the pore formation induced by leukotoxins. Intracellular release of H(2)O(2) induced by fMLP and PMA was not primed by leukotoxins but was inhibited. It seems that leukotoxin inhibition of H(2)O(2) release is independent of pore formation but secondary to an intracellular event, as yet unknown, triggered by leukotoxins.
Figures
Similar articles
-
Analysis of the specificity of Panton-Valentine leucocidin and gamma-hemolysin F component binding.Infect Immun. 2009 Jan;77(1):266-73. doi: 10.1128/IAI.00402-08. Epub 2008 Oct 6. Infect Immun. 2009. PMID: 18838523 Free PMC article.
-
LukM/LukF'-PV is the most active Staphylococcus aureus leukotoxin on bovine neutrophils.Microbes Infect. 2006 Jul;8(8):2068-74. doi: 10.1016/j.micinf.2006.03.004. Epub 2006 May 26. Microbes Infect. 2006. PMID: 16782383
-
Flow cytometric determination of Panton-Valentine leucocidin S component binding.Infect Immun. 2001 Apr;69(4):2390-5. doi: 10.1128/IAI.69.4.2390-2395.2001. Infect Immun. 2001. PMID: 11254598 Free PMC article.
-
Superoxide anion production and phospholipase D-mediated generation of diacylglycerol are subnormal after N-formyl-methionyl-leucyl-phenylalanine stimulation of polymorphonuclear granulocytes in polycythemia vera.J Lab Clin Med. 1993 Feb;121(2):310-9. J Lab Clin Med. 1993. PMID: 8381848
-
Dissociation of mitogen-activated protein kinase activation from the oxidative burst in differentiated HL-60 cells and human neutrophils.J Biol Chem. 1995 Jun 30;270(26):15719-24. doi: 10.1074/jbc.270.26.15719. J Biol Chem. 1995. PMID: 7797573
Cited by
-
Mechanisms of bacterial virulence in pulmonary infections.Curr Opin Crit Care. 2010 Feb;16(1):8-12. doi: 10.1097/MCC.0b013e3283354710. Curr Opin Crit Care. 2010. PMID: 19956071 Free PMC article. Review.
-
Analysis of the specificity of Panton-Valentine leucocidin and gamma-hemolysin F component binding.Infect Immun. 2009 Jan;77(1):266-73. doi: 10.1128/IAI.00402-08. Epub 2008 Oct 6. Infect Immun. 2009. PMID: 18838523 Free PMC article.
-
Staphylococcus aureus pore-forming toxins: The interface of pathogen and host complexity.Semin Cell Dev Biol. 2017 Dec;72:101-116. doi: 10.1016/j.semcdb.2017.04.003. Epub 2017 Apr 23. Semin Cell Dev Biol. 2017. PMID: 28445785 Free PMC article. Review.
-
Sublytic concentrations of Staphylococcus aureus Panton-Valentine leukocidin alter human PMN gene expression and enhance bactericidal capacity.J Leukoc Biol. 2012 Aug;92(2):361-74. doi: 10.1189/jlb.1111575. Epub 2012 May 11. J Leukoc Biol. 2012. PMID: 22581932 Free PMC article.
-
Panton-Valentine Leukocidin associated with S. aureus osteomyelitis activates platelets via neutrophil secretion products.Sci Rep. 2018 Feb 1;8(1):2185. doi: 10.1038/s41598-018-20582-z. Sci Rep. 2018. PMID: 29391581 Free PMC article.
References
-
- Baba-Moussa, L., S. Werner, D. A. Colin, L. Mourey, J.-D. Pédelacq, J. P. Samama, H. Monteil, and G. Prévost. 1999. Discoupling the Ca2+-activation from the pore-forming function of the bi-component Panton-Valentine leucocidin in human PMNs. FEBS Lett. 461:280-286. - PubMed
-
- Bei, L., T. Hu, Z. M. Qian, and X. Shen. 1998. Extracellular Ca2+ regulates the respiratory burst of human neutrophils. Biochim. Biophys. Acta 1404:475-483. - PubMed
-
- Cockeran, R., A. J. Theron, H. C. Steel, N. M. Matlola, T. J. Mitchell, C. Feldman, and R. Anderson. 2001. Proinflammatory interactions of pneumolysin with human neutrophils. J. Infect. Dis. 183:604-611. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

