Mdm30 is an F-box protein required for maintenance of fusion-competent mitochondria in yeast
- PMID: 12808031
- PMCID: PMC194880
- DOI: 10.1091/mbc.e02-12-0831
Mdm30 is an F-box protein required for maintenance of fusion-competent mitochondria in yeast
Abstract
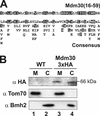
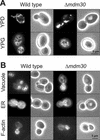
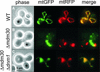
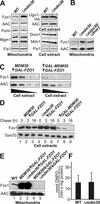
Mitochondrial fusion and fission play important roles for mitochondrial morphology and function. We identified Mdm30 as a novel component required for maintenance of fusion-competent mitochondria in yeast. The Mdm30 sequence contains an F-box motif that is commonly found in subunits of Skp1-Cdc53-F-box protein ubiquitin ligases. A fraction of Mdm30 is associated with mitochondria. Cells lacking Mdm30 contain highly aggregated or fragmented mitochondria instead of the branched tubular network seen in wild-type cells. Deltamdm30 cells lose mitochondrial DNA at elevated temperature and fail to fuse mitochondria in zygotes at all temperatures. These defects are rescued by deletion of DNM1, a gene encoding a component of the mitochondrial division machinery. The protein level of Fzo1, a key component of the mitochondrial fusion machinery, is regulated by Mdm30. Elevated Fzo1 levels in cells lacking Mdm30 or in cells overexpressing Fzo1 from a heterologous promoter induce mitochondrial aggregation in a similar manner. Our results suggest that Mdm30 controls mitochondrial shape by regulating the steady-state level of Fzo1 and point to a connection of the ubiquitin/26S proteasome system and mitochondria.
Figures



Similar articles
-
Regulation of mitochondrial fusion by the F-box protein Mdm30 involves proteasome-independent turnover of Fzo1.J Cell Biol. 2006 Jun 5;173(5):645-50. doi: 10.1083/jcb.200512079. Epub 2006 May 30. J Cell Biol. 2006. PMID: 16735578 Free PMC article.
-
Nonredundant roles of mitochondria-associated F-box proteins Mfb1 and Mdm30 in maintenance of mitochondrial morphology in yeast.Mol Biol Cell. 2006 Sep;17(9):3745-55. doi: 10.1091/mbc.e06-01-0053. Epub 2006 Jun 21. Mol Biol Cell. 2006. PMID: 16790496 Free PMC article.
-
Sequential requirements for the GTPase domain of the mitofusin Fzo1 and the ubiquitin ligase SCFMdm30 in mitochondrial outer membrane fusion.J Cell Sci. 2011 May 1;124(Pt 9):1403-10. doi: 10.1242/jcs.079293. J Cell Sci. 2011. PMID: 21502136 Free PMC article.
-
Mitochondrial dynamics and disease, OPA1.Biochim Biophys Acta. 2006 May-Jun;1763(5-6):500-9. doi: 10.1016/j.bbamcr.2006.04.003. Epub 2006 Apr 20. Biochim Biophys Acta. 2006. PMID: 16737747 Review.
-
Biosynthesis and roles of phospholipids in mitochondrial fusion, division and mitophagy.Cell Mol Life Sci. 2014 Oct;71(19):3767-78. doi: 10.1007/s00018-014-1648-6. Epub 2014 May 28. Cell Mol Life Sci. 2014. PMID: 24866973 Free PMC article. Review.
Cited by
-
Mitofusin-mediated contacts between mitochondria and peroxisomes regulate mitochondrial fusion.PLoS Biol. 2024 Apr 26;22(4):e3002602. doi: 10.1371/journal.pbio.3002602. eCollection 2024 Apr. PLoS Biol. 2024. PMID: 38669296 Free PMC article.
-
E4 ubiquitin ligase promotes mitofusin turnover and mitochondrial stress response.Mol Cell. 2023 Aug 17;83(16):2976-2990.e9. doi: 10.1016/j.molcel.2023.07.021. Mol Cell. 2023. PMID: 37595558 Free PMC article.
-
Docking and stability defects in mitofusin highlight the proteasome as a potential therapeutic target.iScience. 2023 Jun 7;26(7):107014. doi: 10.1016/j.isci.2023.107014. eCollection 2023 Jul 21. iScience. 2023. PMID: 37416455 Free PMC article.
-
Mrz1, a Novel Mitochondrial Outer Membrane RING Finger Protein, is Degraded Through the Ubiquitin-Proteasome Pathway in Schizosaccharomyces pombe.Curr Microbiol. 2022 Sep 10;79(10):309. doi: 10.1007/s00284-022-02998-z. Curr Microbiol. 2022. PMID: 36088506
-
Schizosaccharomyces pombe Fzo1 is subjected to the ubiquitin-proteasome-mediated degradation during the stationary phase.Int Microbiol. 2022 May;25(2):397-404. doi: 10.1007/s10123-022-00231-2. Epub 2022 Jan 25. Int Microbiol. 2022. PMID: 35075549
References
-
- Bähler, J., Wu, J.Q., Longtine, M.S., Shah, N.G., McKenzie, A., 3rd, Steever, A.B., Wach, A., Philippsen, P., and Pringle, J.R. (1998). Heterologous modules for efficient and versatile PCR-based gene targeting in Schizosaccharomyces pombe. Yeast 14, 943-951. - PubMed
-
- Bai, C., Sen, P., Hofmann, K., Ma, L., Goebl, M., Harper, J.W., and Elledge, S.J. (1996). SKP1 connects cell cycle regulators to the ubiquitin proteolysis machinery through a novel motif, the F-box. Cell 86, 263-274. - PubMed
-
- Bereiter-Hahn, J., and Vöth, M. (1994). Dynamics of mitochondria in living cells: shape changes, dislocations, fusion, and fission of mitochondria. Microsc. Res. Tech. 27, 198-219. - PubMed
-
- Berger, K.H., and Yaffe, M.P. (2000). Mitochondrial DNA inheritance in Saccharomyces cerevisiae. Trends Microbiol. 8, 508-513. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

