Tomographic evidence for continuous turnover of Golgi cisternae in Pichia pastoris
- PMID: 12808029
- PMCID: PMC260745
- DOI: 10.1091/mbc.e02-10-0697
Tomographic evidence for continuous turnover of Golgi cisternae in Pichia pastoris
Abstract
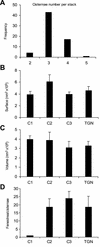
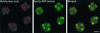
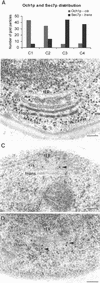
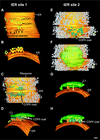
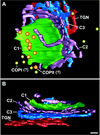
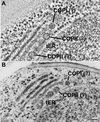
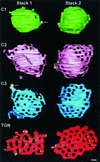
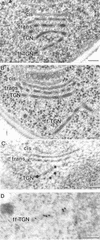
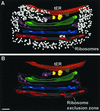
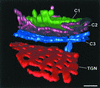
The budding yeast Pichia pastoris contains ordered Golgi stacks next to discrete transitional endoplasmic reticulum (tER) sites, making this organism ideal for structure-function studies of the secretory pathway. Here, we have used P. pastoris to test various models for Golgi trafficking. The experimental approach was to analyze P. pastoris tER-Golgi units by using cryofixed and freeze-substituted cells for electron microscope tomography, immunoelectron microscopy, and serial thin section analysis of entire cells. We find that tER sites and the adjacent Golgi stacks are enclosed in a ribosome-excluding "matrix." Each stack contains three to four cisternae, which can be classified as cis, medial, trans, or trans-Golgi network (TGN). No membrane continuities between compartments were detected. This work provides three major new insights. First, two types of transport vesicles accumulate at the tER-Golgi interface. Morphological analysis indicates that the center of the tER-Golgi interface contains COPII vesicles, whereas the periphery contains COPI vesicles. Second, fenestrae are absent from cis cisternae, but are present in medial through TGN cisternae. The number and distribution of the fenestrae suggest that they form at the edges of the medial cisternae and then migrate inward. Third, intact TGN cisternae apparently peel off from the Golgi stacks and persist for some time in the cytosol, and these "free-floating" TGN cisternae produce clathrin-coated vesicles. These observations are most readily explained by assuming that Golgi cisternae form at the cis face of the stack, progressively mature, and ultimately dissociate from the trans face of the stack.
Figures




Similar articles
-
The yeast GRASP Grh1 colocalizes with COPII and is dispensable for organizing the secretory pathway.Traffic. 2010 Sep;11(9):1168-79. doi: 10.1111/j.1600-0854.2010.01089.x. Epub 2010 Jun 21. Traffic. 2010. PMID: 20573068 Free PMC article. Review.
-
Golgi structure correlates with transitional endoplasmic reticulum organization in Pichia pastoris and Saccharomyces cerevisiae.J Cell Biol. 1999 Apr 5;145(1):69-81. doi: 10.1083/jcb.145.1.69. J Cell Biol. 1999. PMID: 10189369 Free PMC article.
-
The transitional ER localization mechanism of Pichia pastoris Sec12.Dev Cell. 2004 May;6(5):649-59. doi: 10.1016/s1534-5807(04)00129-7. Dev Cell. 2004. PMID: 15130490
-
De novo formation of transitional ER sites and Golgi structures in Pichia pastoris.Nat Cell Biol. 2002 Oct;4(10):750-6. doi: 10.1038/ncb852. Nat Cell Biol. 2002. PMID: 12360285
-
A cisternal maturation mechanism can explain the asymmetry of the Golgi stack.FEBS Lett. 1997 Sep 8;414(2):177-81. doi: 10.1016/s0014-5793(97)00984-8. FEBS Lett. 1997. PMID: 9315681 Review.
Cited by
-
Golgi inheritance in small buds of Saccharomyces cerevisiae is linked to endoplasmic reticulum inheritance.Proc Natl Acad Sci U S A. 2004 Dec 28;101(52):18018-23. doi: 10.1073/pnas.0408256102. Epub 2004 Dec 13. Proc Natl Acad Sci U S A. 2004. PMID: 15596717 Free PMC article.
-
Design of a novel switchable antibody display system in Pichia pastoris.Appl Microbiol Biotechnol. 2022 Sep;106(18):6209-6224. doi: 10.1007/s00253-022-12108-5. Epub 2022 Aug 12. Appl Microbiol Biotechnol. 2022. PMID: 35953606
-
Quantifying Golgi structure using EM: combining volume-SEM and stereology for higher throughput.Histochem Cell Biol. 2017 Jun;147(6):653-669. doi: 10.1007/s00418-017-1564-6. Epub 2017 Apr 20. Histochem Cell Biol. 2017. PMID: 28429122 Free PMC article. Review.
-
Mechanisms and Regulation of the Mitotic Inheritance of the Golgi Complex.Front Cell Dev Biol. 2015 Dec 16;3:79. doi: 10.3389/fcell.2015.00079. eCollection 2015. Front Cell Dev Biol. 2015. PMID: 26734607 Free PMC article. Review.
-
The yeast GRASP Grh1 colocalizes with COPII and is dispensable for organizing the secretory pathway.Traffic. 2010 Sep;11(9):1168-79. doi: 10.1111/j.1600-0854.2010.01089.x. Epub 2010 Jun 21. Traffic. 2010. PMID: 20573068 Free PMC article. Review.
References
-
- Barlowe, C. (2002). COPII-dependent transport from the endoplasmic reticulum. Curr. Opin. Cell Biol. 14, 417-422. - PubMed
-
- Berger, E.G., and Roth, J.P.D.D. (1997). The Golgi Apparatus. Boston: Birkhäuser Verlag.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

