STAT protein interference and suppression of cytokine signal transduction by measles virus V protein
- PMID: 12805463
- PMCID: PMC164804
- DOI: 10.1128/jvi.77.13.7635-7644.2003
STAT protein interference and suppression of cytokine signal transduction by measles virus V protein
Abstract
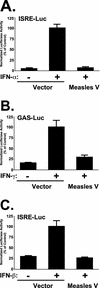
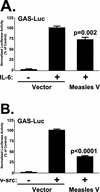
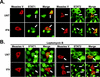
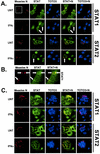
Measles virus, a paramyxovirus of the Morbillivirus genus, is responsible for an acute childhood illness that infects over 40 million people and leads to the deaths of more than 1 million people annually (C. J. Murray and A. D. Lopez, Lancet 349:1269-1276, 1997). Measles virus infection is characterized by virus-induced immune suppression that creates susceptibility to opportunistic infections. Here we demonstrate that measles virus can inhibit cytokine responses by direct interference with host STAT protein-dependent signaling systems. Expression of the measles V protein prevents alpha, beta, and gamma interferon-induced transcriptional responses. Furthermore, it can interfere with signaling by interleukin-6 and the non-receptor tyrosine kinase, v-Src. Affinity purification demonstrates that the measles V protein associates with cellular STAT1, STAT2, STAT3, and IRF9, as well as several unidentified partners. Mechanistic studies indicate that while the measles V protein does not interfere with STAT1 or STAT2 tyrosine phosphorylation, it causes a defect in IFN-induced STAT nuclear accumulation. The defective STAT nuclear redistribution is also observed in measles virus-infected cells, where some of the STAT protein is detected in cytoplasmic bodies that contain viral nucleocapsid protein and nucleic acids. Interference with STAT-inducible transcription may provide a novel intracellular mechanism for measles virus-induced cytokine inhibition that links innate immune evasion to adaptive immune suppression.
Figures


Similar articles
-
STAT2 is a primary target for measles virus V protein-mediated alpha/beta interferon signaling inhibition.J Virol. 2008 Sep;82(17):8330-8. doi: 10.1128/JVI.00831-08. Epub 2008 Jun 25. J Virol. 2008. PMID: 18579593 Free PMC article.
-
The Measles Virus V Protein Binding Site to STAT2 Overlaps That of IRF9.J Virol. 2020 Aug 17;94(17):e01169-20. doi: 10.1128/JVI.01169-20. Print 2020 Aug 17. J Virol. 2020. PMID: 32581091 Free PMC article.
-
Nipah virus V protein evades alpha and gamma interferons by preventing STAT1 and STAT2 activation and nuclear accumulation.J Virol. 2002 Nov;76(22):11476-83. doi: 10.1128/jvi.76.22.11476-11483.2002. J Virol. 2002. PMID: 12388709 Free PMC article.
-
Measles virus V protein blocks interferon (IFN)-alpha/beta but not IFN-gamma signaling by inhibiting STAT1 and STAT2 phosphorylation.FEBS Lett. 2003 Jun 19;545(2-3):177-82. doi: 10.1016/s0014-5793(03)00528-3. FEBS Lett. 2003. PMID: 12804771
-
Weapons of STAT destruction. Interferon evasion by paramyxovirus V protein.Eur J Biochem. 2004 Dec;271(23-24):4621-8. doi: 10.1111/j.1432-1033.2004.04425.x. Eur J Biochem. 2004. PMID: 15606749 Review.
Cited by
-
Adenosine deaminase acting on RNA 1 (ADAR1) suppresses the induction of interferon by measles virus.J Virol. 2012 Apr;86(7):3787-94. doi: 10.1128/JVI.06307-11. Epub 2012 Jan 25. J Virol. 2012. PMID: 22278222 Free PMC article.
-
Measles virus, immune control, and persistence.FEMS Microbiol Rev. 2012 May;36(3):649-62. doi: 10.1111/j.1574-6976.2012.00330.x. Epub 2012 Mar 13. FEMS Microbiol Rev. 2012. PMID: 22316382 Free PMC article. Review.
-
Attenuated rabies virus activates, while pathogenic rabies virus evades, the host innate immune responses in the central nervous system.J Virol. 2005 Oct;79(19):12554-65. doi: 10.1128/JVI.79.19.12554-12565.2005. J Virol. 2005. PMID: 16160183 Free PMC article.
-
Viral suppression of the interferon system.Biochimie. 2007 Jun-Jul;89(6-7):836-42. doi: 10.1016/j.biochi.2007.01.005. Epub 2007 Jan 27. Biochimie. 2007. PMID: 17336442 Free PMC article. Review.
-
Differential cellular immune responses to wild-type and attenuated edmonston tag measles virus strains are primarily defined by the viral phosphoprotein gene.J Med Virol. 2010 Nov;82(11):1966-75. doi: 10.1002/jmv.21899. J Med Virol. 2010. PMID: 20872725 Free PMC article.
References
-
- Aaronson, D. S., and C. M. Horvath. 2002. A road map for those who don't know JAK-STAT. Science 296:1653-1655. - PubMed
-
- Bohn, W., F. Ciampor, R. Rutter, and K. Mannweiler. 1990. Localization of nucleocapsid associated polypeptides in measles virus-infected cells by immunogold labelling after resin embedding. Arch. Virol. 114:53-64. - PubMed
-
- Bolt, G., K. Berg, and M. Blixenkrone-Moller. 2002. Measles virus-induced modulation of host-cell gene expression. J. Gen. Virol. 83:1157-1165. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

