Cleavage of von Willebrand factor requires the spacer domain of the metalloprotease ADAMTS13
- PMID: 12791682
- PMCID: PMC11033693
- DOI: 10.1074/jbc.M305331200
Cleavage of von Willebrand factor requires the spacer domain of the metalloprotease ADAMTS13
Abstract
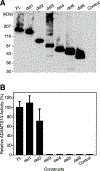
ADAMTS13 consists of a reprolysin-type metalloprotease domain followed by a disintegrin domain, a thrombospondin type 1 motif (TSP1), Cys-rich and spacer domains, seven more TSP1 motifs, and two CUB domains. ADAMTS13 limits platelet accumulation in microvascular thrombi by cleaving the Tyr1605-Met1606 bond in von Willebrand factor, and ADAMTS13 deficiency causes a lethal syndrome, thrombotic thrombocytopenic purpura. ADAMTS13 domains required for substrate recognition were localized by the characterization of recombinant deletion mutants. Constructs with C-terminal His6 and V5 epitopes were expressed by transient transfection of COS-7 cells or in a baculovirus system. No association with extracellular matrix or cell surface was detected for any ADAMTS13 variant by immunofluorescence microscopy or chemical modification. Both plasma and recombinant full-length ADAMTS13 cleaved von Willebrand factor subunits into two fragments of 176 kDa and 140 kDa. Recombinant ADAMTS13 was divalent metal ion-dependent and was inhibited by IgG from a patient with idiopathic thrombotic thrombocytopenic purpura. ADAMTS13 that was truncated after the metalloprotease domain, the disintegrin domain, the first TSP1 repeat, or the Cys-rich domain was not able to cleave von Willebrand factor, whereas addition of the spacer region restored protease activity. Therefore, the spacer region is necessary for normal ADAMTS13 activity toward von Willebrand factor, and the more C-terminal TSP1 and CUB domains are dispensable in vitro.
Figures




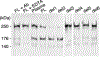

Similar articles
-
Molecular biology of ADAMTS13 and diagnostic utility of ADAMTS13 proteolytic activity and inhibitor assays.Semin Thromb Hemost. 2005 Dec;31(6):659-72. doi: 10.1055/s-2005-925472. Semin Thromb Hemost. 2005. PMID: 16388417 Free PMC article. Review.
-
The proximal carboxyl-terminal domains of ADAMTS13 determine substrate specificity and are all required for cleavage of von Willebrand factor.J Biol Chem. 2005 Aug 19;280(33):29428-34. doi: 10.1074/jbc.M505513200. Epub 2005 Jun 23. J Biol Chem. 2005. PMID: 15975930 Free PMC article.
-
Binding of ADAMTS13 to von Willebrand factor.J Biol Chem. 2005 Jun 10;280(23):21773-8. doi: 10.1074/jbc.M502529200. Epub 2005 Apr 11. J Biol Chem. 2005. PMID: 15824096
-
ADAMTS-13 cysteine-rich/spacer domains are functionally essential for von Willebrand factor cleavage.Blood. 2003 Nov 1;102(9):3232-7. doi: 10.1182/blood-2003-03-0908. Epub 2003 Jul 17. Blood. 2003. PMID: 12869506
-
Interplay between ADAMTS13 and von Willebrand factor in inherited and acquired thrombotic microangiopathies.Semin Hematol. 2005 Jan;42(1):56-62. doi: 10.1053/j.seminhematol.2004.09.008. Semin Hematol. 2005. PMID: 15662617 Review.
Cited by
-
ADAMTS13 Biomarkers in Management of Immune Thrombotic Thrombocytopenic Purpura.Arch Pathol Lab Med. 2023 Aug 1;147(8):974-979. doi: 10.5858/arpa.2022-0050-RA. Arch Pathol Lab Med. 2023. PMID: 36223210 Free PMC article. Review.
-
Further characterization of ADAMTS-13 inactivation by thrombin.J Thromb Haemost. 2007 May;5(5):1010-8. doi: 10.1111/j.1538-7836.2007.02514.x. Epub 2007 Mar 12. J Thromb Haemost. 2007. PMID: 17355572 Free PMC article.
-
Molecular biology of ADAMTS13 and diagnostic utility of ADAMTS13 proteolytic activity and inhibitor assays.Semin Thromb Hemost. 2005 Dec;31(6):659-72. doi: 10.1055/s-2005-925472. Semin Thromb Hemost. 2005. PMID: 16388417 Free PMC article. Review.
-
Pathogenesis of thrombotic microangiopathies.Annu Rev Pathol. 2008;3:249-77. doi: 10.1146/annurev.pathmechdis.3.121806.154311. Annu Rev Pathol. 2008. PMID: 18215115 Free PMC article. Review.
-
Essential domains of a disintegrin and metalloprotease with thrombospondin type 1 repeats-13 metalloprotease required for modulation of arterial thrombosis.Arterioscler Thromb Vasc Biol. 2011 Oct;31(10):2261-9. doi: 10.1161/ATVBAHA.111.229609. Epub 2011 Jul 28. Arterioscler Thromb Vasc Biol. 2011. PMID: 21799176 Free PMC article.
References
-
- George JN (2000) Blood 96, 1223–1229 - PubMed
-
- Furlan M, and Lämmle B (2001) Best Pract. Res. Clin. Haematol 14, 437–454 - PubMed
-
- Moake JL (2002) Annu. Rev. Med 53, 75–88 - PubMed
-
- Rock GA, Shumak KH, Buskard NA, Blanchette VS, Kelton JG, Nair RC, and Spasoff RA (1991) N. Engl. J. Med 325, 393–397 - PubMed
-
- Moake JL, Rudy CK, Troll JH, Weinstein MJ, Colannino NM, Azocar J, Seder RH, Hong SL, and Deykin D (1982) N. Engl. J. Med 307, 1432–1435 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

