PML colocalizes with and stabilizes the DNA damage response protein TopBP1
- PMID: 12773567
- PMCID: PMC156140
- DOI: 10.1128/MCB.23.12.4247-4256.2003
PML colocalizes with and stabilizes the DNA damage response protein TopBP1
Abstract
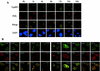
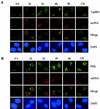
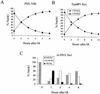
The PML tumor suppressor gene is consistently disrupted by t(15;17) in patients with acute promyelocytic leukemia. Promyelocytic leukemia protein (PML) is a multifunctional protein that plays essential roles in cell growth regulation, apoptosis, transcriptional regulation, and genome stability. Our study here shows that PML colocalizes and associates in vivo with the DNA damage response protein TopBP1 in response to ionizing radiation (IR). Both PML and TopBP1 colocalized with the IR-induced bromodeoxyuridine single-stranded DNA foci. PML and TopBP1 also colocalized with Rad50, Brca1, ATM, Rad9, and BLM. IR and interferon (IFN) coinduce the expression levels of both TopBP1 and PML. In PML-deficient NB4 cells, TopBP1 was unable to form IR-induced foci. All-trans-retinoic acid induced reorganization of the PML nuclear body (NB) and reappearance of the IR-induced TopBP1 foci. Inhibition of PML expression by siRNA is associated with a significant decreased in TopBP1 expression. Furthermore, PML-deficient cells express a low level of TopBP1, and its expression cannot be induced by IR or IFN. Adenovirus-mediated overexpression of PML in PML(-/-) mouse embryo fibroblasts substantially increased TopBP1 expression, which colocalized with the PML NBs. These studies demonstrated a mechanism of PML-dependent expression of TopBP1. PML overexpression induced TopBP1 protein but not the mRNA expression. Pulse-chase labeling analysis demonstrated that PML overexpression stabilized the TopBP1 protein, suggesting that PML plays a role in regulating the stability of TopBP1 in response to IR. Together, our findings demonstrate that PML regulates TopBP1 functions by association and stabilization of the protein in response to IR-induced DNA damage.
Figures


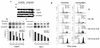

Similar articles
-
Promyelocytic leukemia protein PML inhibits Nur77-mediated transcription through specific functional interactions.Oncogene. 2002 May 30;21(24):3925-33. doi: 10.1038/sj.onc.1205491. Oncogene. 2002. PMID: 12032831
-
Cell-cycle regulation of DNA damage-induced expression of the suppressor gene PML.Biochem Biophys Res Commun. 1997 Nov 26;240(3):640-6. doi: 10.1006/bbrc.1997.7692. Biochem Biophys Res Commun. 1997. PMID: 9398618
-
Regulation and localization of the Bloom syndrome protein in response to DNA damage.J Cell Biol. 2001 Apr 16;153(2):367-80. doi: 10.1083/jcb.153.2.367. J Cell Biol. 2001. PMID: 11309417 Free PMC article.
-
Ret finger protein is a normal component of PML nuclear bodies and interacts directly with PML.J Cell Sci. 1998 May;111 ( Pt 10):1319-29. doi: 10.1242/jcs.111.10.1319. J Cell Sci. 1998. PMID: 9570750
-
Role and fate of PML nuclear bodies in response to interferon and viral infections.Oncogene. 2001 Oct 29;20(49):7274-86. doi: 10.1038/sj.onc.1204854. Oncogene. 2001. PMID: 11704856 Review.
Cited by
-
Assembly of checkpoint and repair machineries at DNA damage sites.Trends Biochem Sci. 2010 Feb;35(2):101-8. doi: 10.1016/j.tibs.2009.09.001. Epub 2009 Oct 28. Trends Biochem Sci. 2010. PMID: 19875294 Free PMC article. Review.
-
ND10 components relocate to sites associated with herpes simplex virus type 1 nucleoprotein complexes during virus infection.J Virol. 2005 Apr;79(8):5078-89. doi: 10.1128/JVI.79.8.5078-5089.2005. J Virol. 2005. PMID: 15795293 Free PMC article.
-
Nesprin-2-dependent ERK1/2 compartmentalisation regulates the DNA damage response in vascular smooth muscle cell ageing.Cell Death Differ. 2015 Sep;22(9):1540-50. doi: 10.1038/cdd.2015.12. Epub 2015 Mar 6. Cell Death Differ. 2015. PMID: 25744025 Free PMC article.
-
PML-like subnuclear bodies, containing XRCC1, juxtaposed to DNA replication-based single-strand breaks.FASEB J. 2019 Feb;33(2):2301-2313. doi: 10.1096/fj.201801379R. Epub 2018 Sep 27. FASEB J. 2019. PMID: 30260704 Free PMC article.
-
SUMOylation of HMGA2: selective destabilization of promyelocytic leukemia protein via proteasome.Mol Cancer Ther. 2008 Apr;7(4):923-34. doi: 10.1158/1535-7163.MCT-07-0540. Mol Cancer Ther. 2008. PMID: 18413806 Free PMC article.
References
-
- Carbone, R., M. Pearson, S. Minucci, and P. P. Pelicci. 2002. PML NBs associate with hMre11 complex and p53at sites of irradiation-induced DNA damage. Oncogene 21:1633-1640. - PubMed
-
- Coultas, L., and A. Strasser. 2000. The molecular control of DNA damage-induced cell death. Apoptosis 5:491-507. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
