Regulation and substrate specificity of the SR protein kinase Clk/Sty
- PMID: 12773558
- PMCID: PMC156123
- DOI: 10.1128/MCB.23.12.4139-4149.2003
Regulation and substrate specificity of the SR protein kinase Clk/Sty
Abstract
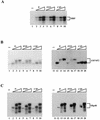
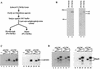
SR proteins constitute a family of splicing factors that play key roles in both constitutive and regulated splicing in metazoan organisms. The proteins are extensively phosphorylated, and kinases capable of phosphorylating them have been identified. However, little is known about how these kinases function, for example, whether they target specific SR proteins or whether the kinases themselves are regulated. Here we describe properties of one such kinase, Clk/Sty, the founding member of the Clk/Sty family of dual-specificity kinases. Clk/Sty is autophosphorylated on both Ser/Thr and Thr residues, and using both direct kinase assays and SR protein-dependent splicing assays, we have analyzed the effects of each type of modification. We find not only that the pattern of phosphorylation on a specific SR protein substrate, ASF/SF2, is modulated by autophosphorylation but also that the ability of Clk/Sty to recognize different SR proteins is influenced by the extent and nature of autophosphorylation. Strikingly, phosphorylation of ASF/SF2 is sensitive to changes in Tyr, but not Ser/Thr, autophosphorylation while that of SC35 displays the opposite pattern. In contrast, phosphorylation of a third SR protein, SRp40, is unaffected by autophosphorylation. We also present biochemical data indicating that as expected for a factor directly involved in splicing control (but in contrast to recent reports), Clk/Sty is found in the nucleus of several different cell types.
Figures




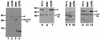
Similar articles
-
The Clk/Sty protein kinase phosphorylates SR splicing factors and regulates their intranuclear distribution.EMBO J. 1996 Jan 15;15(2):265-75. EMBO J. 1996. PMID: 8617202 Free PMC article.
-
SRPK1 and Clk/Sty protein kinases show distinct substrate specificities for serine/arginine-rich splicing factors.J Biol Chem. 1996 Oct 4;271(40):24569-75. doi: 10.1074/jbc.271.40.24569. J Biol Chem. 1996. PMID: 8798720
-
Interplay between SRPK and Clk/Sty kinases in phosphorylation of the splicing factor ASF/SF2 is regulated by a docking motif in ASF/SF2.Mol Cell. 2005 Oct 7;20(1):77-89. doi: 10.1016/j.molcel.2005.08.025. Mol Cell. 2005. PMID: 16209947
-
Human CDC2-like kinase 1 (CLK1): a novel target for Alzheimer's disease.Curr Drug Targets. 2014 May;15(5):539-50. doi: 10.2174/1389450115666140226112321. Curr Drug Targets. 2014. PMID: 24568585 Review.
-
Cdc-Like Kinases (CLKs): Biology, Chemical Probes, and Therapeutic Potential.Int J Mol Sci. 2020 Oct 13;21(20):7549. doi: 10.3390/ijms21207549. Int J Mol Sci. 2020. PMID: 33066143 Free PMC article. Review.
Cited by
-
Regiospecific phosphorylation control of the SR protein ASF/SF2 by SRPK1.J Mol Biol. 2009 Jul 24;390(4):618-34. doi: 10.1016/j.jmb.2009.05.060. Epub 2009 May 27. J Mol Biol. 2009. PMID: 19477182 Free PMC article.
-
Cdc2-like kinases: structure, biological function, and therapeutic targets for diseases.Signal Transduct Target Ther. 2023 Apr 7;8(1):148. doi: 10.1038/s41392-023-01409-4. Signal Transduct Target Ther. 2023. PMID: 37029108 Free PMC article. Review.
-
A CLK1-KKT2 Signaling Pathway Regulating Kinetochore Assembly in Trypanosoma brucei.mBio. 2021 Jun 29;12(3):e0068721. doi: 10.1128/mBio.00687-21. Epub 2021 Jun 15. mBio. 2021. PMID: 34128702 Free PMC article.
-
Processive phosphorylation: mechanism and biological importance.Cell Signal. 2007 Nov;19(11):2218-26. doi: 10.1016/j.cellsig.2007.06.006. Epub 2007 Jun 22. Cell Signal. 2007. PMID: 17644338 Free PMC article. Review.
-
Clks 1, 2 and 4 prevent chromatin breakage by regulating the Aurora B-dependent abscission checkpoint.Nat Commun. 2016 Apr 29;7:11451. doi: 10.1038/ncomms11451. Nat Commun. 2016. PMID: 27126587 Free PMC article.
References
-
- Chang, L., and M. Karin. 2001. Mammalian MAP kinase signalling cascades. Nature 410:37-40. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
