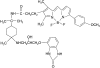
Pharmacology and direct visualisation of BODIPY-TMR-CGP: a long-acting fluorescent beta2-adrenoceptor agonist
- PMID: 12770928
- PMCID: PMC1573863
- DOI: 10.1038/sj.bjp.0705287
Pharmacology and direct visualisation of BODIPY-TMR-CGP: a long-acting fluorescent beta2-adrenoceptor agonist
Abstract
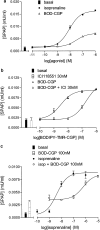
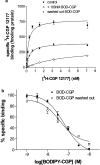
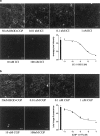
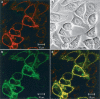
1 Fluorescence techniques offer a way to circumvent several problems associated with many radioligand binding and functional assays and the need for large numbers of cells. Fluorescent ligands also offer the advantage of allowing real time direct visualisation of ligand - receptors interactions. A fluorescent analogue of CGP 12177 (BODIPY-TMR-CGP) has thus been evaluated as a beta(2)-adrenoceptor ligand in CHO-K1 cells expressing the human beta(2)-adrenoceptor. 2 Studies of (3)H-cAMP accumulation showed that BODIPY-TMR-CGP stimulated an increase in cAMP accumulation and cyclic AMP response element (CRE)-mediated gene transcription with an EC(50) of 21-28 nM. Both of these responses were antagonised by the selective beta(2)-adrenoceptor antagonist ICI 118551. 3 Binding studies with (3)H-CGP 12177, and functional studies of CRE-regulated gene transcription showed that the BODIPY-TMR-CGP interaction with the human beta(2)-adrenoceptor is of very long duration. 4 Visualisation of the binding of BODIPY-TMR-CGP to single living mammalian cells was clearly demonstrated by confocal microscopy and showed that this ligand was able to selectively label cell surface beta(2)-adrenoceptors in living CHO-K1 cells transfected with the human beta(2)-adrenoceptor with an apparent K(D) of 27 nM. Studies with cells expressing a beta(2)-adrenoceptor-green fluorescent protein (GFP) fusion protein provided further strong evidence that BODIPY-TMR-CGP was binding to the beta(2)-adrenoceptor. 5 BODIPY-TMR-CGP is therefore a long-acting fluorescent beta(2)-adrenoceptor agonist that can be used to label beta(2)-adrenoceptors in the plasma membrane of living cells.
Figures



Similar articles
-
Synthesis and characterization of high-affinity 4,4-difluoro-4-bora-3a,4a-diaza-s-indacene-labeled fluorescent ligands for human β-adrenoceptors.J Med Chem. 2011 Oct 13;54(19):6874-87. doi: 10.1021/jm2008562. Epub 2011 Sep 16. J Med Chem. 2011. PMID: 21870877 Free PMC article.
-
Detection of the secondary, low-affinity β1 -adrenoceptor site in living cells using the fluorescent CGP 12177 derivative BODIPY-TMR-CGP.Br J Pharmacol. 2014 Dec;171(23):5431-45. doi: 10.1111/bph.12858. Br J Pharmacol. 2014. PMID: 25052258 Free PMC article.
-
Pharmacological characterization of CGP 12177 at the human beta(2)-adrenoceptor.Br J Pharmacol. 2002 Oct;137(3):400-8. doi: 10.1038/sj.bjp.0704855. Br J Pharmacol. 2002. PMID: 12237261 Free PMC article.
-
Negative cooperativity across β1-adrenoceptor homodimers provides insights into the nature of the secondary low-affinity CGP 12177 β1-adrenoceptor binding conformation.FASEB J. 2015 Jul;29(7):2859-71. doi: 10.1096/fj.14-265199. Epub 2015 Apr 2. FASEB J. 2015. PMID: 25837585 Free PMC article.
-
Human heart beta-adrenoceptors: beta1-adrenoceptor diversification through 'affinity states' and polymorphism.Clin Exp Pharmacol Physiol. 2007 Oct;34(10):1020-8. doi: 10.1111/j.1440-1681.2007.04730.x. Clin Exp Pharmacol Physiol. 2007. PMID: 17714089 Review.
Cited by
-
Fluorescent ligand binding reveals heterogeneous distribution of adrenoceptors and 'cannabinoid-like' receptors in small arteries.Br J Pharmacol. 2010 Feb;159(4):787-96. doi: 10.1111/j.1476-5381.2009.00608.x. Epub 2010 Feb 5. Br J Pharmacol. 2010. PMID: 20136833 Free PMC article.
-
Fluorescence- and bioluminescence-based approaches to study GPCR ligand binding.Br J Pharmacol. 2016 Oct;173(20):3028-37. doi: 10.1111/bph.13316. Epub 2015 Nov 5. Br J Pharmacol. 2016. PMID: 26317175 Free PMC article. Review.
-
Different β-adrenoceptor subtypes coupling to cAMP or NO/cGMP pathways: implications in the relaxant response of rat conductance and resistance vessels.Br J Pharmacol. 2013 May;169(2):413-25. doi: 10.1111/bph.12121. Br J Pharmacol. 2013. PMID: 23373597 Free PMC article.
-
Complex Formation between VEGFR2 and the β2-Adrenoceptor.Cell Chem Biol. 2019 Jun 20;26(6):830-841.e9. doi: 10.1016/j.chembiol.2019.02.014. Epub 2019 Apr 4. Cell Chem Biol. 2019. PMID: 30956148 Free PMC article.
-
Synthesis and characterization of high-affinity 4,4-difluoro-4-bora-3a,4a-diaza-s-indacene-labeled fluorescent ligands for human β-adrenoceptors.J Med Chem. 2011 Oct 13;54(19):6874-87. doi: 10.1021/jm2008562. Epub 2011 Sep 16. J Med Chem. 2011. PMID: 21870877 Free PMC article.
References
-
- BAKER J.G., HALL I.P., HILL S.J.Pharmacology and direct visualization of the fluorescent β2-adrenoceptor ligand BODIPY-CGP 12177 in CHO cells transfected with the human β2-adrenoceptor Br. J. Pharmacol. 200113450p
-
- COLEMAN R.A., JOHNSON M., NIALS A.T., VARDEY C.J. Exosites: their current status, and their relevance to the duration of action of long-acting beta 2-adrenoceptor agonists. Trends Pharmacol. Sci. 1996;17:324–330. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

