An autoregulatory loop controls peroxisome proliferator-activated receptor gamma coactivator 1alpha expression in muscle
- PMID: 12764228
- PMCID: PMC165838
- DOI: 10.1073/pnas.1232352100
An autoregulatory loop controls peroxisome proliferator-activated receptor gamma coactivator 1alpha expression in muscle
Abstract
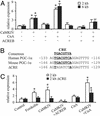
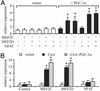
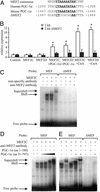
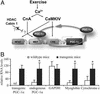
Skeletal muscle adapts to chronic physical activity by inducing mitochondrial biogenesis and switching proportions of muscle fibers from type II to type I. Several major factors involved in this process have been identified, such as the calcium/calmodulin-dependent protein kinase IV (CaMKIV), calcineurin A (CnA), and the transcriptional component peroxisome proliferator-activated receptor gamma coactivator 1alpha (PGC-1alpha). Transgenic expression of PGC-1alpha recently has been shown to dramatically increase the content of type I muscle fibers in skeletal muscle, but the relationship between PGC-1alpha expression and the key components in calcium signaling is not clear. In this report, we show that the PGC-1alpha promoter is regulated by both CaMKIV and CnA activity. CaMKIV activates PGC-1alpha largely through the binding of cAMP response element-binding protein to the PGC-1alpha promoter. Moreover, we show that a positive feedback loop exists between PGC-1alpha and members of the myocyte enhancer factor 2 (MEF2) family of transcription factors. MEF2s bind to the PGC-1alpha promoter and activate it, predominantly when coactivated by PGC-1alpha. MEF2 activity is stimulated further by CnA signaling. These findings imply a unified pathway, integrating key regulators of calcium signaling with the transcriptional switch PGC-1alpha. Furthermore, these data suggest an autofeedback loop whereby the calcium-signaling pathway may result in a stable induction of PGC-1alpha, contributing to the relatively stable nature of muscle fiber-type determination.
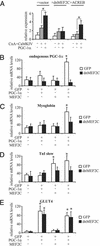
Figures
Similar articles
-
Calcineurin A and CaMKIV transactivate PGC-1alpha promoter, but differentially regulate cytochrome c promoter in rat skeletal muscle.Pflugers Arch. 2007 May;454(2):297-305. doi: 10.1007/s00424-007-0206-6. Epub 2007 Feb 2. Pflugers Arch. 2007. PMID: 17273866
-
Real-time imaging of peroxisome proliferator-activated receptor-gamma coactivator-1alpha promoter activity in skeletal muscles of living mice.Am J Physiol Cell Physiol. 2004 Sep;287(3):C790-6. doi: 10.1152/ajpcell.00425.2003. Epub 2004 May 19. Am J Physiol Cell Physiol. 2004. PMID: 15151904
-
MEF2 responds to multiple calcium-regulated signals in the control of skeletal muscle fiber type.EMBO J. 2000 May 2;19(9):1963-73. doi: 10.1093/emboj/19.9.1963. EMBO J. 2000. PMID: 10790363 Free PMC article.
-
Adaptation of Skeletal Muscles to Contractile Activity of Varying Duration and Intensity: The Role of PGC-1α.Biochemistry (Mosc). 2018 Jun;83(6):613-628. doi: 10.1134/S0006297918060019. Biochemistry (Mosc). 2018. PMID: 30195320 Review.
-
Transcriptional control of the Pgc-1alpha gene in skeletal muscle in vivo.Exerc Sport Sci Rev. 2007 Jul;35(3):97-101. doi: 10.1097/JES.0b013e3180a03169. Exerc Sport Sci Rev. 2007. PMID: 17620927 Review.
Cited by
-
Calcineurin A and CaMKIV transactivate PGC-1alpha promoter, but differentially regulate cytochrome c promoter in rat skeletal muscle.Pflugers Arch. 2007 May;454(2):297-305. doi: 10.1007/s00424-007-0206-6. Epub 2007 Feb 2. Pflugers Arch. 2007. PMID: 17273866
-
Activation of cardiac Cdk9 represses PGC-1 and confers a predisposition to heart failure.EMBO J. 2004 Sep 1;23(17):3559-69. doi: 10.1038/sj.emboj.7600351. Epub 2004 Aug 5. EMBO J. 2004. PMID: 15297879 Free PMC article.
-
Regulation of Age-related Decline by Transcription Factors and Their Crosstalk with the Epigenome.Curr Genomics. 2018 Sep;19(6):464-482. doi: 10.2174/1389202919666180503125850. Curr Genomics. 2018. PMID: 30258277 Free PMC article. Review.
-
Transducer of regulated CREB-binding proteins (TORCs) induce PGC-1alpha transcription and mitochondrial biogenesis in muscle cells.Proc Natl Acad Sci U S A. 2006 Sep 26;103(39):14379-84. doi: 10.1073/pnas.0606714103. Epub 2006 Sep 15. Proc Natl Acad Sci U S A. 2006. PMID: 16980408 Free PMC article.
-
Perm1 regulates CaMKII activation and shapes skeletal muscle responses to endurance exercise training.Mol Metab. 2019 May;23:88-97. doi: 10.1016/j.molmet.2019.02.009. Epub 2019 Feb 27. Mol Metab. 2019. PMID: 30862473 Free PMC article.
References
-
- Booth, F. W. & Thomason, D. B. (1991) Physiol. Rev. 71, 541-585. - PubMed
-
- Berchtold, M. W., Brinkmeier, H. & Muntener, M. (2000) Physiol. Rev. 80, 1215-1265. - PubMed
-
- Olson, E. N. & Williams, R. S. (2000) Cell 101, 689-692. - PubMed
-
- Hood, D. A. (2001) J. Appl. Physiol. 90, 1137-1157. - PubMed
-
- Corcoran, E. E. & Means, A. R. (2001) J. Biol. Chem. 276, 2975-2978. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

