Hypoxia-inducible erythropoietin signaling in squamous dysplasia and squamous cell carcinoma of the uterine cervix and its potential role in cervical carcinogenesis and tumor progression
- PMID: 12759237
- PMCID: PMC1868129
- DOI: 10.1016/S0002-9440(10)64314-3
Hypoxia-inducible erythropoietin signaling in squamous dysplasia and squamous cell carcinoma of the uterine cervix and its potential role in cervical carcinogenesis and tumor progression
Abstract
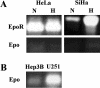
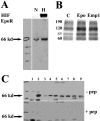
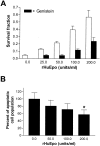
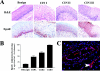
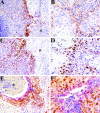
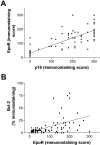
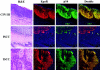
Tissue hypoxia is a characteristic property of cervical cancers that makes tumors resistant to chemo- and radiation therapy. Erythropoietin (Epo) is a hypoxia-inducible stimulator of erythropoiesis. Acting via its receptor (EpoR), Epo up-regulates bcl-2 and inhibits apoptosis of erythroid cells and rescues neurons from hypoxic damage. In addition to human papillomavirus infection, increased bcl-2 expression and decreased apoptosis are thought to play a role in the progression of cervical neoplasia. Using reverse transcriptase-polymerase chain reaction and Western blotting we showed that HeLa and SiHa cervical carcinoma cells and human cervical carcinomas express EpoR, and that hypoxia enhances EpoR expression. Exogenous Epo stimulated tyrosine phosphorylation and inhibited the cytotoxic effect of cisplatin in HeLa cervical carcinoma cells. Using immunohistochemistry, we examined the expression of Epo, EpoR, p16, hypoxia-inducible factor (HIF)-1alpha, and bcl-2 in benign and dysplastic cervical squamous epithelia and invasive squamous cell carcinomas (ISCCs). EpoR expression in benign epithelia was confined to the basal cell layers, whereas in dysplasias it increasingly appeared in more superficial cell layers and showed a significant correlation with severity of dysplasia. Diffuse EpoR expression was found in all ISCCs. Expression of Epo and HIF-1alpha was increased in dysplasias compared to benign epithelia. Focal Epo and HIF-1alpha expression was seen near necrotic areas in ISCCs, and showed correlation in their spatial distribution. Significant correlation was found between expression of EpoR, and p16 and bcl-2 in benign and dysplastic squamous epithelia. Our results suggest that increased expression of Epo and EpoR may play a significant role in cervical carcinogenesis and tumor progression. Hypoxia-inducible Epo signaling may play a significant role in the aggressive behavior and treatment resistance of hypoxic cervical cancers.
Figures




Similar articles
-
Prognostic significance of erythropoietin expression in human endometrial carcinoma.Cancer. 2004 Jun 1;100(11):2376-86. doi: 10.1002/cncr.20244. Cancer. 2004. PMID: 15160341
-
Roles for hypoxia-regulated genes during cervical carcinogenesis: somatic evolution during the hypoxia-glycolysis-acidosis sequence.Gynecol Oncol. 2008 Feb;108(2):377-84. doi: 10.1016/j.ygyno.2007.10.034. Epub 2007 Dec 4. Gynecol Oncol. 2008. PMID: 18055005
-
Expression of erythropoietin and its receptor increases in colonic neoplastic progression: the role of hypoxia in tumorigenesis.Indian J Pathol Microbiol. 2011 Apr-Jun;54(2):273-8. doi: 10.4103/0377-4929.81591. Indian J Pathol Microbiol. 2011. PMID: 21623073
-
How does hypoxia inducible factor-1α participate in enhancing the glycolysis activity in cervical cancer?Ann Diagn Pathol. 2013 Jun;17(3):305-11. doi: 10.1016/j.anndiagpath.2012.12.002. Epub 2013 Feb 1. Ann Diagn Pathol. 2013. PMID: 23375385 Review.
-
Mystery Story about Erythropoietin (Epo) and Erythropoietin Receptor (EpoR) are Disguised?Hepatogastroenterology. 2015 May;62(139):585-9. Hepatogastroenterology. 2015. PMID: 26897933 Review.
Cited by
-
Role of the erythropoietin receptor in Lung Cancer cells: erythropoietin exhibits angiogenic potential.J Cancer. 2020 Aug 21;11(20):6090-6100. doi: 10.7150/jca.36924. eCollection 2020. J Cancer. 2020. PMID: 32922549 Free PMC article.
-
Long-term results of radiation therapy oncology group 9903: a randomized phase 3 trial to assess the effect of erythropoietin on local-regional control in anemic patients treated with radiation therapy for squamous cell carcinoma of the head and neck.Int J Radiat Oncol Biol Phys. 2015 Apr 1;91(5):907-15. doi: 10.1016/j.ijrobp.2014.12.018. Epub 2015 Feb 7. Int J Radiat Oncol Biol Phys. 2015. PMID: 25670542 Free PMC article. Clinical Trial.
-
Long-term and stable correction of uremic anemia by intramuscular injection of plasmids containing hypoxia-regulated system of erythropoietin expression.Exp Mol Med. 2012 Nov 30;44(11):674-83. doi: 10.3858/emm.2012.44.11.076. Exp Mol Med. 2012. PMID: 22990115 Free PMC article.
-
Hypoxia promotes the proliferation of cervical carcinoma cells through stimulating the secretion of IL-8.Int J Clin Exp Pathol. 2014 Jan 15;7(2):575-83. eCollection 2014. Int J Clin Exp Pathol. 2014. PMID: 24551277 Free PMC article.
-
Prognostic significance of erythropoietin and erythropoietin receptor in gastric adenocarcinoma.World J Gastroenterol. 2011 Sep 14;17(34):3933-40. doi: 10.3748/wjg.v17.i34.3933. World J Gastroenterol. 2011. PMID: 22025882 Free PMC article.
References
-
- Rose PG: Chemoradiotherapy for cervical cancer. Eur J Cancer 2002, 38:270-278 - PubMed
-
- Advances in the treatment of cervical cancer. 20April2001. International Network for Cancer Treatment and Research, Brussels, Belgium Meeting
-
- Parkin DM, Pisani P, Ferlay J: Global cancer statistics. CA Cancer J Clin 1999, 49:31-64 - PubMed
-
- Parkin DM, Pisani P, Ferlay J: Estimates of the worldwide incidence of 25 major cancers in 1990. Int J Cancer 1999, 80:827-841 - PubMed
-
- Greenlee RT, Murray T, Bolden S, Wingo PA: Cancer statistics, 2000. CA Cancer J Clin 2000, 50:7-33 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

