Herpes simplex virus type 1 portal protein UL6 interacts with the putative terminase subunits UL15 and UL28
- PMID: 12743292
- PMCID: PMC154995
- DOI: 10.1128/jvi.77.11.6351-6358.2003
Herpes simplex virus type 1 portal protein UL6 interacts with the putative terminase subunits UL15 and UL28
Abstract
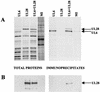
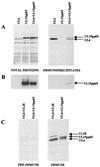
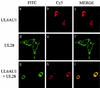
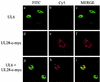
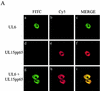
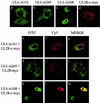
The herpes simplex virus type 1 (HSV-1) UL6, UL15, and UL28 proteins are essential for cleavage of replicated concatemeric viral DNA into unit length genomes and their packaging into a preformed icosahedral capsid known as the procapsid. The capsid-associated UL6 DNA-packaging protein is located at a single vertex and is thought to form the portal through which the genome enters the procapsid. The UL15 protein interacts with the UL28 protein, and both are strong candidates for subunits of the viral terminase, a key component of the molecular motor that drives the DNA into the capsid. To investigate the association of the UL6 protein with the UL15 and UL28 proteins, the three proteins were produced in large amounts in insect cells with the baculovirus expression system. Interactions between UL6 and UL28 and between UL6 and UL15 were identified by an immunoprecipitation assay. These results were confirmed by transiently expressing wild-type and mutant proteins in mammalian cells and monitoring their distribution by immunofluorescence. In cells expressing the single proteins, UL6 and UL15 were concentrated in the nuclei whereas UL28 was found in the cytoplasm. When the UL6 and UL28 proteins were coexpressed, UL28 was redistributed to the nuclei, where it colocalized with UL6. In cells producing either of two cytoplasmic UL6 mutant proteins and a functional epitope-tagged form of UL15, the UL15 protein was concentrated with the mutant UL6 protein in the cytoplasm. These observed interactions of UL6 with UL15 and UL28 are likely to be of major importance in establishing a functional DNA-packaging complex at the portal vertex of the HSV-1 capsid.
Figures

Similar articles
-
Linker insertion mutations in the herpes simplex virus type 1 UL28 gene: effects on UL28 interaction with UL15 and UL33 and identification of a second-site mutation in the UL15 gene that suppresses a lethal UL28 mutation.J Virol. 2006 Dec;80(24):12312-23. doi: 10.1128/JVI.01766-06. Epub 2006 Oct 11. J Virol. 2006. PMID: 17035316 Free PMC article.
-
Herpes simplex virus type 1 cleavage and packaging proteins UL15 and UL28 are associated with B but not C capsids during packaging.J Virol. 1998 Sep;72(9):7428-39. doi: 10.1128/JVI.72.9.7428-7439.1998. J Virol. 1998. PMID: 9696839 Free PMC article.
-
Interaction of the herpes simplex virus type 1 packaging protein UL15 with full-length and deleted forms of the UL28 protein.J Gen Virol. 2000 Dec;81(Pt 12):2999-3009. doi: 10.1099/0022-1317-81-12-2999. J Gen Virol. 2000. PMID: 11086131
-
Herpesvirus Capsid Assembly and DNA Packaging.Adv Anat Embryol Cell Biol. 2017;223:119-142. doi: 10.1007/978-3-319-53168-7_6. Adv Anat Embryol Cell Biol. 2017. PMID: 28528442 Free PMC article. Review.
-
Involvement of Terminase Complex in Herpes Simplex Virus Mature Virion Egress.Curr Protein Pept Sci. 2022;23(2):105-113. doi: 10.2174/1389203723666220217144432. Curr Protein Pept Sci. 2022. PMID: 35176987 Review.
Cited by
-
The putative herpes simplex virus 1 chaperone protein UL32 modulates disulfide bond formation during infection.J Virol. 2015 Jan;89(1):443-53. doi: 10.1128/JVI.01913-14. Epub 2014 Oct 15. J Virol. 2015. PMID: 25320327 Free PMC article.
-
Linker insertion mutations in the herpes simplex virus type 1 UL28 gene: effects on UL28 interaction with UL15 and UL33 and identification of a second-site mutation in the UL15 gene that suppresses a lethal UL28 mutation.J Virol. 2006 Dec;80(24):12312-23. doi: 10.1128/JVI.01766-06. Epub 2006 Oct 11. J Virol. 2006. PMID: 17035316 Free PMC article.
-
Disulfide bond formation in the herpes simplex virus 1 UL6 protein is required for portal ring formation and genome encapsidation.J Virol. 2011 Sep;85(17):8616-24. doi: 10.1128/JVI.00123-11. Epub 2011 May 18. J Virol. 2011. PMID: 21593161 Free PMC article.
-
Divergent Evolution of Nuclear Localization Signal Sequences in Herpesvirus Terminase Subunits.J Biol Chem. 2016 May 20;291(21):11420-33. doi: 10.1074/jbc.M116.724393. Epub 2016 Mar 31. J Biol Chem. 2016. PMID: 27033706 Free PMC article.
-
DNA methyltransferase DNMT3A associates with viral proteins and impacts HSV-1 infection.Proteomics. 2015 Jun;15(12):1968-82. doi: 10.1002/pmic.201500035. Epub 2015 May 7. Proteomics. 2015. PMID: 25758154 Free PMC article.
References
-
- Abbotts, A. P., V. G. Preston, M. Hughes, A. H. Patel, and N. D. Stow. 2000. Interaction of the herpes simplex virus type 1 packaging protein UL15 with full-length and deleted forms of the UL28 protein. J. Gen. Virol. 81:2999-3009. - PubMed
-
- Addison, C., F. J. Rixon, J. W. Palfreyman, M. Ohara, and V. G. Preston. 1984. Characterization of a herpes simplex virus type-1 mutant which has a temperature-sensitive defect in penetration of cells and assembly of capsids. Virology 138:246-259. - PubMed
-
- Addison, C., F. J. Rixon, and V. G. Preston. 1990. Herpes simplex virus type-1 UL28 gene product is important for the formation of mature capsids. J. Gen. Virol. 71:2377-2384. - PubMed
-
- Al-Kobaisi, M. F., F. J. Rixon, I. McDougall, and V. G. Preston. 1991. The herpes simplex virus UL33 gene-product is required for the assembly of full capsids. Virology 180:380-388. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

