The nuclear pore complex protein ALADIN is mislocalized in triple A syndrome
- PMID: 12730363
- PMCID: PMC156285
- DOI: 10.1073/pnas.1031047100
The nuclear pore complex protein ALADIN is mislocalized in triple A syndrome
Abstract
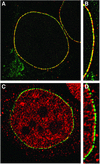
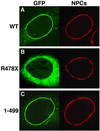
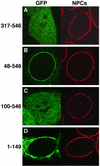
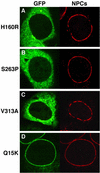
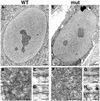
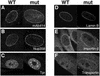
Triple A syndrome is a human autosomal recessive disorder characterized by an unusual array of tissue-specific defects. Triple A syndrome arises from mutations in a WD-repeat protein of unknown function called ALADIN (also termed Adracalin or AAAS). We showed previously that ALADIN localizes to nuclear pore complexes (NPCs), large multiprotein assemblies that are the sole sites of nucleocytoplasmic transport. Here, we present evidence indicating that NPC targeting is essential for the function of ALADIN. Characterization of mutant ALADIN proteins from triple A patients revealed a striking effect of these mutations on NPC targeting. A variety of disease-associated missense, nonsense, and frameshift mutations failed to localize to NPCs and were found predominantly in the cytoplasm. Microscopic analysis of cells from a triple A patient revealed no morphological abnormalities of the nuclei, nuclear envelopes, or NPCs. Importantly, these findings indicate that defects in NPC function, rather than structure, give rise to triple A syndrome. We propose that ALADIN plays a cell type-specific role in regulating nucleocytoplasmic transport and that this function is essential for the proper maintenance andor development of certain tissues. Our findings provide a foundation for understanding the molecular basis of triple A syndrome and may lead to unique insights into the role of nucleocytoplasmic transport in adrenal function and neurodevelopment.
Figures
Similar articles
-
Cellular localization of 17 natural mutant variants of ALADIN protein in triple A syndrome - shedding light on an unexpected splice mutation.Biochem Cell Biol. 2006 Apr;84(2):243-9. doi: 10.1139/o05-198. Biochem Cell Biol. 2006. PMID: 16609705
-
[From gene to disease; adrenocortical insufficiency, achalasia and disrupted tear secretion: Allgrove syndrome].Ned Tijdschr Geneeskd. 2002 Nov 30;146(48):2295-7. Ned Tijdschr Geneeskd. 2002. PMID: 12497758 Review. Dutch.
-
The transmembrane nucleoporin NDC1 is required for targeting of ALADIN to nuclear pore complexes.Biochem Biophys Res Commun. 2009 Nov 6;389(1):100-4. doi: 10.1016/j.bbrc.2009.08.096. Epub 2009 Aug 22. Biochem Biophys Res Commun. 2009. PMID: 19703420
-
The triple A syndrome is due to mutations in ALADIN, a novel member of the nuclear pore complex.Endocr Res. 2004 Nov;30(4):891-9. doi: 10.1081/erc-200044138. Endocr Res. 2004. PMID: 15666842
-
Triple-A Syndrome (TAS): An In-Depth Overview on Genetic and Phenotype Heterogeneity.Protein Pept Lett. 2020;27(12):1192-1203. doi: 10.2174/0929866527666200613215449. Protein Pept Lett. 2020. PMID: 32533814 Review.
Cited by
-
Nuclear Envelope and Nuclear Pore Complexes in Neurodegenerative Diseases-New Perspectives for Therapeutic Interventions.Mol Neurobiol. 2021 Mar;58(3):983-995. doi: 10.1007/s12035-020-02168-x. Epub 2020 Oct 17. Mol Neurobiol. 2021. PMID: 33067781 Free PMC article. Review.
-
ALADINI482S causes selective failure of nuclear protein import and hypersensitivity to oxidative stress in triple A syndrome.Proc Natl Acad Sci U S A. 2006 Feb 14;103(7):2298-303. doi: 10.1073/pnas.0505598103. Epub 2006 Feb 7. Proc Natl Acad Sci U S A. 2006. PMID: 16467144 Free PMC article.
-
Diseases of the nuclear envelope.Cold Spring Harb Perspect Biol. 2010 Feb;2(2):a000760. doi: 10.1101/cshperspect.a000760. Cold Spring Harb Perspect Biol. 2010. PMID: 20182615 Free PMC article. Review.
-
Mechanosensing at the Nuclear Envelope by Nuclear Pore Complex Stretch Activation and Its Effect in Physiology and Pathology.Front Physiol. 2019 Jul 12;10:896. doi: 10.3389/fphys.2019.00896. eCollection 2019. Front Physiol. 2019. PMID: 31354529 Free PMC article.
-
Nuclear pore dysfunction and disease: a complex opportunity.Nucleus. 2024 Dec;15(1):2314297. doi: 10.1080/19491034.2024.2314297. Epub 2024 Feb 21. Nucleus. 2024. PMID: 38383349 Free PMC article. Review.
References
-
- Allgrove J, Clayden G S, Grant D B, Macaulay J C. Lancet. 1978;1:1284–1286. - PubMed
-
- Clark A J, Weber A. Endocr Rev. 1998;19:828–843. - PubMed
-
- Tullio-Pelet A, Salomon R, Hadj-Rabia S, Mugnier C, de Laet M H, Chaouachi B, Bakiri F, Brottier P, Cattolico L, Penet C, et al. Nat Genet. 2000;26:332–335. - PubMed
-
- Handschug K, Sperling S, Yoon S J, Hennig S, Clark A J, Huebner A. Hum Mol Genet. 2001;10:283–290. - PubMed
-
- Smith T F, Gaitatzes C, Saxena K, Neer E J. Trends Biochem Sci. 1999;24:181–185. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

