Stimulation of preadipocyte differentiation by steroid through targeting of an HDAC1 complex
- PMID: 12727880
- PMCID: PMC156090
- DOI: 10.1093/emboj/cdg218
Stimulation of preadipocyte differentiation by steroid through targeting of an HDAC1 complex
Abstract
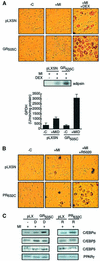
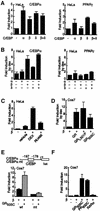
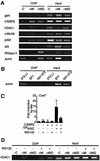
Glucocorticoids potentiate the early steps of preadipocyte differentiation and promote obesity in Cushing's syndrome and during prolonged steroid therapy. We show that glucocorticoids stimulate 3T3 L1 preadipocyte differentiation through a non-transcriptional mechanism mediated through the ligand-binding domain of the glucocorticoid receptor. This enhanced the onset of CCAAT/enhancer binding protein (C/EBPalpha) expression by potentiating its initial transcriptional activation by C/EBPbeta. In the absence of steroid, C/EBPbeta associated with a transcriptional corepressor complex containing mSin3A and histone deacetylase 1 (HDAC1), but lacking HDAC2 and RbAp46/48. HDAC1/mSin3A were recruited to the C/EBPalpha promoter with C/EBPbeta and promoted the deacetylation of histone H4. Steroid induced the specific depletion of this corepressor by targeting the HDAC1 within the complex for degradation through the 26S proteasome. Treatment with histone deacetylase inhibitors replaced the effects of steroid treatment on preadipocyte differentiation and C/EBPalpha expression, while overexpression of HDAC1 abrogated the stimulatory effects of steroid. Recapitulation of the glucocorticoid effect by progestin treatment in the presence of the progesterone receptor ligand-binding domain suggests a conserved mechanism relevant to many aspects of steroid-mediated differentiation.
Figures



Similar articles
-
Glucocorticoid-stimulated preadipocyte differentiation is mediated through acetylation of C/EBPbeta by GCN5.Proc Natl Acad Sci U S A. 2007 Feb 20;104(8):2703-8. doi: 10.1073/pnas.0607378104. Epub 2007 Feb 14. Proc Natl Acad Sci U S A. 2007. PMID: 17301242 Free PMC article.
-
A positive regulatory domain in CCAAT/enhancer binding protein β (C/EBPΒ) is required for the glucocorticoid-mediated displacement of histone deacetylase 1 (HDAC1) from the C/ebpα promoter and maximum adipogenesis.Endocrinology. 2013 Apr;154(4):1454-64. doi: 10.1210/en.2012-2061. Epub 2013 Mar 1. Endocrinology. 2013. PMID: 23456364
-
Inactivation of histone deacetylase 1 (HDAC1) but not HDAC2 is required for the glucocorticoid-dependent CCAAT/enhancer-binding protein α (C/EBPα) expression and preadipocyte differentiation.Endocrinology. 2014 Dec;155(12):4762-73. doi: 10.1210/en.2014-1565. Epub 2014 Sep 9. Endocrinology. 2014. PMID: 25203139
-
Modulation of early human preadipocyte differentiation by glucocorticoids.Endocrinology. 2006 Nov;147(11):5284-93. doi: 10.1210/en.2006-0267. Epub 2006 Jul 27. Endocrinology. 2006. PMID: 16873539
-
Liver-enriched inhibitory protein (LIP) actively inhibits preadipocyte differentiation through histone deacetylase 1 (HDAC1).J Biol Chem. 2011 Jun 17;286(24):21488-99. doi: 10.1074/jbc.M110.211540. Epub 2011 Apr 25. J Biol Chem. 2011. PMID: 21521687 Free PMC article.
Cited by
-
Cidea is an essential transcriptional coactivator regulating mammary gland secretion of milk lipids.Nat Med. 2012 Jan 15;18(2):235-43. doi: 10.1038/nm.2614. Nat Med. 2012. PMID: 22245780
-
Rapid mechanisms of glucocorticoid signaling in the Leydig cell.Steroids. 2008 Oct;73(9-10):1018-24. doi: 10.1016/j.steroids.2007.12.020. Epub 2007 Dec 28. Steroids. 2008. PMID: 18281069 Free PMC article. Review.
-
C/EBP maintains chromatin accessibility in liver and facilitates glucocorticoid receptor recruitment to steroid response elements.EMBO J. 2013 May 29;32(11):1568-83. doi: 10.1038/emboj.2013.106. Epub 2013 May 10. EMBO J. 2013. PMID: 23665916 Free PMC article.
-
ATF6 modulates SREBP2-mediated lipogenesis.EMBO J. 2004 Feb 25;23(4):950-8. doi: 10.1038/sj.emboj.7600106. Epub 2004 Feb 5. EMBO J. 2004. PMID: 14765107 Free PMC article.
-
Transcription factor Smad3 is required for the inhibition of adipogenesis by retinoic acid.J Biol Chem. 2010 Apr 23;285(17):13274-84. doi: 10.1074/jbc.M109.054536. Epub 2010 Feb 23. J Biol Chem. 2010. PMID: 20179325 Free PMC article.
References
-
- Boruk M., Savory,J.G. and Hache,R.J. (1998) AF-2-dependent potentiation of CCAAT enhancer binding protein β-mediated transcriptional activation by glucocorticoid receptor. Mol. Endocrinol., 12, 1749–1763. - PubMed
-
- Cao Z., Umek,R.M. and McKnight,S.L. (1991) Regulated expression of three C/EBP isoforms during adipose conversion of 3T3-L1 cells. Genes Dev., 5, 1538–1552. - PubMed
-
- Davis R., Peters,D.H. and McTavish,D. (1994) Valproic acid. A reappraisal of its pharmacological properties and clinical efficacy in epilepsy. Drugs, 47, 332–372. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

