CD4+CD25+ immunoregulatory T cells may not be involved in controlling autoimmune arthritis
- PMID: 12718754
- PMCID: PMC165034
- DOI: 10.1186/ar624
CD4+CD25+ immunoregulatory T cells may not be involved in controlling autoimmune arthritis
Abstract
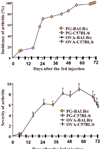
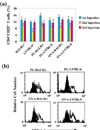
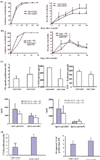
Accumulating evidence suggests that regulatory T cells play a crucial role in preventing autoimmunity. Recently, a naturally occurring CD4+CD25+ T-cell subset that is anergic and also suppressive has been shown to suppress autoimmunity in several animal models. We used proteoglycan-induced arthritis (PGIA) as a study model to investigate the role of the CD4+CD25+ regulatory T cells in autoimmune arthritis. There was no significant change in the percentage of CD4+CD25+ T cells during the immunization period when proteoglycan- or ovalbumin-immunized BALB/c and C57BL/6 mice were compared. An adoptive transfer study showed that the CD4+CD25+ T cells did not protect severe combined immunodeficient mice from arthritis when they were cotransferred with splenocytes from arthritic animals. Similarly, depletion of the CD4+CD25+ T cells did not enhance the onset of the disease or disease severity in severe combined immunodeficient mice. Moreover, CD28-deficient mice, which have very few CD4+CD25+ T cells, were highly resistant to PGIA. These findings indicate that the CD4+CD25+ regulatory T cells may not play a critical role in controlling PGIA.
Figures

Similar articles
-
Thymus and autoimmunity: production of CD25+CD4+ naturally anergic and suppressive T cells as a key function of the thymus in maintaining immunologic self-tolerance.J Immunol. 1999 May 1;162(9):5317-26. J Immunol. 1999. PMID: 10228007
-
Immunologic self-tolerance maintained by CD25+CD4+ naturally anergic and suppressive T cells: induction of autoimmune disease by breaking their anergic/suppressive state.Int Immunol. 1998 Dec;10(12):1969-80. doi: 10.1093/intimm/10.12.1969. Int Immunol. 1998. PMID: 9885918
-
Naturally anergic and suppressive CD25(+)CD4(+) T cells as a functionally and phenotypically distinct immunoregulatory T cell subpopulation.Int Immunol. 2000 Aug;12(8):1145-55. doi: 10.1093/intimm/12.8.1145. Int Immunol. 2000. PMID: 10917889
-
Autoimmune ovarian disease in day 3-thymectomized mice: the neonatal time window, antigen specificity of disease suppression, and genetic control.Curr Top Microbiol Immunol. 2005;293:209-47. doi: 10.1007/3-540-27702-1_10. Curr Top Microbiol Immunol. 2005. PMID: 15981482 Review.
-
The role of regulatory T lymphocytes in asthma pathogenesis.Curr Allergy Asthma Rep. 2005 Mar;5(2):136-41. doi: 10.1007/s11882-005-0087-8. Curr Allergy Asthma Rep. 2005. PMID: 15683614 Review.
Cited by
-
Circulating CD4+CD25+ T regulatory cells are not altered in multiple sclerosis and unaffected by disease-modulating drugs.J Clin Immunol. 2004 Mar;24(2):155-61. doi: 10.1023/B:JOCI.0000019780.93817.82. J Clin Immunol. 2004. PMID: 15024182 Clinical Trial.
-
Efficient Therapeutic Function and Mechanisms of Human Polyclonal CD8+CD103+Foxp3+ Regulatory T Cells on Collagen-Induced Arthritis in Mice.J Immunol Res. 2019 Feb 19;2019:8575407. doi: 10.1155/2019/8575407. eCollection 2019. J Immunol Res. 2019. PMID: 30915372 Free PMC article.
-
TGF-β-Induced Regulatory T Cells Directly Suppress B Cell Responses through a Noncytotoxic Mechanism.J Immunol. 2016 May 1;196(9):3631-41. doi: 10.4049/jimmunol.1501740. Epub 2016 Mar 21. J Immunol. 2016. PMID: 27001954 Free PMC article.
-
Delineating the role of the HLA-DR4 "shared epitope" in susceptibility versus resistance to develop arthritis.J Immunol. 2008 Aug 15;181(4):2869-77. doi: 10.4049/jimmunol.181.4.2869. J Immunol. 2008. PMID: 18684978 Free PMC article.
-
Therapeutic potential of TGF-β-induced CD4(+) Foxp3(+) regulatory T cells in autoimmune diseases.Autoimmunity. 2011 Feb;44(1):43-50. doi: 10.3109/08916931003782163. Epub 2010 Jul 29. Autoimmunity. 2011. PMID: 20670119 Free PMC article. Review.
References
-
- Ohashi PS. T-cell signaling and autoimmunity: molecular mechanisms of disease. Nat Rev Immunol. 2002;2:427–438. - PubMed
-
- Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–1164. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

