A reelin-integrin receptor interaction regulates Arc mRNA translation in synaptoneurosomes
- PMID: 12707415
- PMCID: PMC154370
- DOI: 10.1073/pnas.1031602100
A reelin-integrin receptor interaction regulates Arc mRNA translation in synaptoneurosomes
Abstract
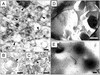
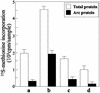
Reelin is synthesized and secreted into extracellular matrix by cortical gamma-aminobutyric acid (GABA)ergic interneurons and binds with high affinity to the extracellular domain of integrin receptors expressed in dendritic shaft and spine postsynaptic densities (DSPSD) of pyramidal neurons. In heterozygous reeler mice, reelin bound to DSPSD, and the expression of Arc (activity-regulated cytoskeletal protein) is lower than in wild-type mice. We studied the effect of reelin on Arc and total protein synthesis in synaptoneurosomes (SNSs) prepared from mouse neocortex. Recombinant full-length mouse reelin displaces the high affinity (K(D) = 60 fM) binding of [(125)I]echistatin (a competitive integrin receptor antagonist) to integrin receptors with a K(i) of 22 pM and with a Hill slope close to 1. Echistatin (50-100 nM) competitively antagonizes and abates reelin binding. The addition of reelin (2-40 pM) to SNSs enhances the incorporation of [(35)S]methionine into Arc and other rapidly translated proteins in a concentration-dependent manner. This incorporation is virtually abolished by 50-100 nM echistatin or by 5-10 nM rapamycin, a blocker of the mammalian target of rapamycin kinase. We conclude that reelin binds with high affinity to integrin receptors expressed in SNSs and thereby activates Arc protein synthesis.
Figures






Similar articles
-
Reelin binds alpha3beta1 integrin and inhibits neuronal migration.Neuron. 2000 Jul;27(1):33-44. doi: 10.1016/s0896-6273(00)00007-6. Neuron. 2000. PMID: 10939329
-
In Patas monkey, glutamic acid decarboxylase-67 and reelin mRNA coexpression varies in a manner dependent on layers and cortical areas.J Comp Neurol. 2002 Sep 23;451(3):279-88. doi: 10.1002/cne.10341. J Comp Neurol. 2002. PMID: 12210139
-
Down-regulation of dendritic spine and glutamic acid decarboxylase 67 expressions in the reelin haploinsufficient heterozygous reeler mouse.Proc Natl Acad Sci U S A. 2001 Mar 13;98(6):3477-82. doi: 10.1073/pnas.051614698. Proc Natl Acad Sci U S A. 2001. PMID: 11248103 Free PMC article.
-
[Role of Reelin during Layer Formation in the Cerebral Neocortex].Brain Nerve. 2016 Aug;68(8):931-7. doi: 10.11477/mf.1416200532. Brain Nerve. 2016. PMID: 27503821 Review. Japanese.
-
REELIN and schizophrenia: a disease at the interface of the genome and the epigenome.Mol Interv. 2002 Feb;2(1):47-57. doi: 10.1124/mi.2.1.47. Mol Interv. 2002. PMID: 14993361 Review.
Cited by
-
Contributions of VLDLR and LRP8 in the establishment of retinogeniculate projections.Neural Dev. 2013 Jun 13;8:11. doi: 10.1186/1749-8104-8-11. Neural Dev. 2013. PMID: 23758727 Free PMC article.
-
DNA methyltransferase 1 regulates reelin mRNA expression in mouse primary cortical cultures.Proc Natl Acad Sci U S A. 2005 Feb 1;102(5):1749-54. doi: 10.1073/pnas.0409648102. Epub 2005 Jan 25. Proc Natl Acad Sci U S A. 2005. PMID: 15671176 Free PMC article.
-
Apoer2-ICD-dependent regulation of hippocampal ribosome mRNA loading.Res Sq [Preprint]. 2023 Jun 28:rs.3.rs-3040567. doi: 10.21203/rs.3.rs-3040567/v1. Res Sq. 2023. Update in: Mol Neurodegener. 2023 Sep 19;18(1):62. doi: 10.1186/s13024-023-00652-1 PMID: 37461529 Free PMC article. Updated. Preprint.
-
Cranial irradiation alters the behaviorally induced immediate-early gene arc (activity-regulated cytoskeleton-associated protein).Cancer Res. 2008 Dec 1;68(23):9763-70. doi: 10.1158/0008-5472.CAN-08-1861. Cancer Res. 2008. PMID: 19047155 Free PMC article.
-
Maternal care effects on the hippocampal transcriptome and anxiety-mediated behaviors in the offspring that are reversible in adulthood.Proc Natl Acad Sci U S A. 2006 Feb 28;103(9):3480-5. doi: 10.1073/pnas.0507526103. Epub 2006 Feb 16. Proc Natl Acad Sci U S A. 2006. PMID: 16484373 Free PMC article.
References
-
- Pappas G D, Kriho V, Pesold C. J Neurocytol. 2001;30:413–425. - PubMed
-
- Costa E, Davis J, Grayson D R, Guidotti A, Pappas G D, Pesold C. Neurobiol Dis. 2000;8:723–742. - PubMed
-
- Rice D S, Curran T. Annu Rev Neurosci. 2001;24:1005–1039. - PubMed
-
- Dulabon L, Olson E C, Taglienti M G, Eisenhuth S, McGrath B, Walsh C A, Kreidberg J A, Anton E S. Neuron. 2000;27:33–44. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

