Transformation of sporozoites into early exoerythrocytic malaria parasites does not require host cells
- PMID: 12707302
- PMCID: PMC2193875
- DOI: 10.1084/jem.20022100
Transformation of sporozoites into early exoerythrocytic malaria parasites does not require host cells
Abstract
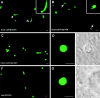
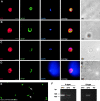
Malaria parasite species that infect mammals, including humans, must first take up residence in hepatic host cells as exoerythrocytic forms (EEF) before initiating infection of red blood cells that leads to malaria disease. Despite the importance of hepatic stages for immunity against malaria, little is known about their biology and antigenic composition. Here, we show that sporozoites, the parasites' transmission stage that resides in the mosquito vector salivary glands, can transform into early EEF without intracellular residence in host hepatocytes. The morphological sequence of transformation and the expression of proteins in the EEF appear indistinguishable from parasites that develop within host cells. Transformation depends on temperature elevation to 37 degrees C and serum. Our findings demonstrate that residence in a host hepatocyte or specific host cell-derived factors are not necessary to bring about the profound morphological and biochemical changes of the parasite that occur after its transmission from vector to mammalian host.
Figures

Similar articles
-
Malaria Parasite Liver Infection and Exoerythrocytic Biology.Cold Spring Harb Perspect Med. 2017 Jun 1;7(6):a025486. doi: 10.1101/cshperspect.a025486. Cold Spring Harb Perspect Med. 2017. PMID: 28242785 Free PMC article. Review.
-
The Puf-family RNA-binding protein Puf2 controls sporozoite conversion to liver stages in the malaria parasite.PLoS One. 2011;6(5):e19860. doi: 10.1371/journal.pone.0019860. Epub 2011 May 18. PLoS One. 2011. PMID: 21673790 Free PMC article.
-
Imaging movement of malaria parasites during transmission by Anopheles mosquitoes.Cell Microbiol. 2004 Jul;6(7):687-94. doi: 10.1111/j.1462-5822.2004.00395.x. Cell Microbiol. 2004. PMID: 15186404
-
Rhoptry neck protein 11 has crucial roles during malaria parasite sporozoite invasion of salivary glands and hepatocytes.Int J Parasitol. 2019 Aug;49(9):725-735. doi: 10.1016/j.ijpara.2019.05.001. Epub 2019 Jun 25. Int J Parasitol. 2019. PMID: 31247198
-
[Mechanisms of liver invasion by malaria sporozoites].Tanpakushitsu Kakusan Koso. 2009 Jun;54(8 Suppl):1029-34. Tanpakushitsu Kakusan Koso. 2009. PMID: 21089536 Review. Japanese. No abstract available.
Cited by
-
Metamorphosis of the malaria parasite in the liver is associated with organelle clearance.Cell Res. 2010 Sep;20(9):1043-59. doi: 10.1038/cr.2010.88. Epub 2010 Jun 22. Cell Res. 2010. PMID: 20567259 Free PMC article.
-
An in vitro assay to measure antibody-mediated inhibition of P. berghei sporozoite invasion against P. falciparum antigens.Sci Rep. 2017 Dec 5;7(1):17011. doi: 10.1038/s41598-017-17274-5. Sci Rep. 2017. PMID: 29209029 Free PMC article.
-
Malaria Parasite Liver Infection and Exoerythrocytic Biology.Cold Spring Harb Perspect Med. 2017 Jun 1;7(6):a025486. doi: 10.1101/cshperspect.a025486. Cold Spring Harb Perspect Med. 2017. PMID: 28242785 Free PMC article. Review.
-
Skin-draining lymph node priming is sufficient to induce sterile immunity against pre-erythrocytic malaria.EMBO Mol Med. 2013 Feb;5(2):250-63. doi: 10.1002/emmm.201201677. Epub 2012 Dec 19. EMBO Mol Med. 2013. PMID: 23255300 Free PMC article.
-
Plasmodium yoelii sporozoites modulate cytokine profile and induce apoptosis in murine Kupffer cells.Int J Parasitol. 2008 Dec;38(14):1639-50. doi: 10.1016/j.ijpara.2008.05.018. Epub 2008 Jul 9. Int J Parasitol. 2008. PMID: 18656478 Free PMC article.
References
-
- Shortt, H.E., and P.C.C. Garnham. 1948. Pre-erythrocytic stage in mammalian malaria parasites. Nature. 161:126. - PubMed
-
- Meis, J.F., J.P. Verhave, P.H. Jap, R.E. Sinden, and J.H. Meuwissen. 1983. Malaria parasites–discovery of the early liver form. Nature. 302:424–426. - PubMed
-
- Meis, J.F., J.P. Verhave, P.H. Jap, and J.H. Meuwissen. 1985. Transformation of sporozoites of Plasmodium berghei into exoerythrocytic forms in the liver of its mammalian host. Cell Tissue Res. 241:353–360. - PubMed
-
- Hollingdale, M.R., and U. Krzych. 2002. Immune responses to liver-stage parasites: implications for vaccine development. Chem. Immunol. 80:97–124. - PubMed
-
- Hoffman, S.L., and D.L. Doolan. 2000. Malaria vaccines-targeting infected hepatocytes. Nat. Med. 6:1218–1219. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

