Adaptation of core mechanisms to generate cell polarity
- PMID: 12700771
- PMCID: PMC3373010
- DOI: 10.1038/nature01602
Adaptation of core mechanisms to generate cell polarity
Abstract
Cell polarity is defined as asymmetry in cell shape, protein distributions and cell functions. It is characteristic of single-cell organisms, including yeast and bacteria, and cells in tissues of multi-cell organisms such as epithelia in worms, flies and mammals. This diversity raises several questions: do different cell types use different mechanisms to generate polarity, how is polarity signalled, how do cells react to that signal, and how is structural polarity translated into specialized functions? Analysis of evolutionarily diverse cell types reveals that cell-surface landmarks adapt core pathways for cytoskeleton assembly and protein transport to generate cell polarity.
Figures

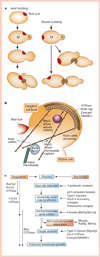
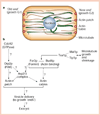
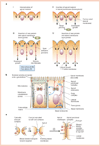
Comment in
-
The cytoskeleton, cellular motility and the reductionist agenda.Nature. 2003 Apr 17;422(6933):741-5. doi: 10.1038/nature01598. Nature. 2003. PMID: 12700767
Similar articles
-
Regulation of epithelial cell shape and polarity by cell-cell adhesion (Review).Mol Membr Biol. 2002 Apr-Jun;19(2):113-20. doi: 10.1080/09687680210137219. Mol Membr Biol. 2002. PMID: 12126229 Review.
-
Structural and regulatory functions of keratins.Exp Cell Res. 2007 Jun 10;313(10):2021-32. doi: 10.1016/j.yexcr.2007.03.005. Epub 2007 Mar 15. Exp Cell Res. 2007. PMID: 17434482 Review.
-
Dynamics and regulation of epithelial adherens junctions: recent discoveries and controversies.Int Rev Cell Mol Biol. 2013;303:27-99. doi: 10.1016/B978-0-12-407697-6.00002-7. Int Rev Cell Mol Biol. 2013. PMID: 23445808 Review.
-
Chapter 3: acquisition of membrane polarity in epithelial tube formation patterns, signaling pathways, molecular mechanisms, and disease.Int Rev Cell Mol Biol. 2009;274:129-82. doi: 10.1016/S1937-6448(08)02003-0. Int Rev Cell Mol Biol. 2009. PMID: 19349037 Review.
-
Mammalian PAR-1 determines epithelial lumen polarity by organizing the microtubule cytoskeleton.J Cell Biol. 2004 Mar 1;164(5):717-27. doi: 10.1083/jcb.200308104. Epub 2004 Feb 23. J Cell Biol. 2004. PMID: 14981097 Free PMC article.
Cited by
-
Prion Protein at the Leading Edge: Its Role in Cell Motility.Int J Mol Sci. 2020 Sep 12;21(18):6677. doi: 10.3390/ijms21186677. Int J Mol Sci. 2020. PMID: 32932634 Free PMC article. Review.
-
The plasma membrane potential and the organization of the actin cytoskeleton of epithelial cells.Int J Cell Biol. 2012;2012:121424. doi: 10.1155/2012/121424. Epub 2012 Jan 23. Int J Cell Biol. 2012. PMID: 22315611 Free PMC article.
-
MARCKS modulates radial progenitor placement, proliferation and organization in the developing cerebral cortex.Development. 2009 Sep;136(17):2965-75. doi: 10.1242/dev.036616. Development. 2009. PMID: 19666823 Free PMC article.
-
Cell polarity signalling at the birth of multicellularity: What can we learn from the first animals.Front Cell Dev Biol. 2022 Nov 24;10:1024489. doi: 10.3389/fcell.2022.1024489. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36506100 Free PMC article. Review.
-
The STE20/germinal center kinase POD6 interacts with the NDR kinase COT1 and is involved in polar tip extension in Neurospora crassa.Mol Biol Cell. 2006 Sep;17(9):4080-92. doi: 10.1091/mbc.e06-01-0072. Epub 2006 Jul 5. Mol Biol Cell. 2006. PMID: 16822837 Free PMC article.
References
-
- Chant J. Cell polarity in yeast. Annu. Rev. Cell Dev. Biol. 1999;15:365–391. - PubMed
-
- Chant J, Herskowitz I. Genetic control of bud site selection in yeast by a set of gene products that constitute a morphogenetic pathway. Cell. 1991;65:1203–1212. - PubMed
-
- Pruyne D, Bretscher A. Polarization of cell growth in yeast I. Establishment and maintenance of polarity states. J. Cell Sci. 2000;113:365–375. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

