The Grb10/Nedd4 complex regulates ligand-induced ubiquitination and stability of the insulin-like growth factor I receptor
- PMID: 12697834
- PMCID: PMC153198
- DOI: 10.1128/MCB.23.9.3363-3372.2003
The Grb10/Nedd4 complex regulates ligand-induced ubiquitination and stability of the insulin-like growth factor I receptor
Abstract
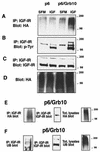
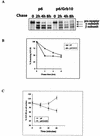
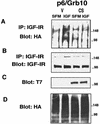
The adapter protein Grb10 belongs to a superfamily of related proteins, including Grb7, -10, and -14 and Caenorhabditis elegans Mig10. Grb10 is an interacting partner of the insulin-like growth factor I receptor (IGF-IR) and the insulin receptor (IR). Previous work showed an inhibitory effect of mouse Grb10 (mGrb10alpha) on IGF-I-mediated mitogenesis (A. Morrione et al., J. Biol. Chem. 272:26382-26387, 1997). With mGrb10alpha as bait in a yeast two-hybrid screen, mouse Nedd4 (mNedd4-1), a ubiquitin protein ligase, was previously isolated as an interacting protein of Grb10 (A. Morrione et al., J. Biol. Chem. 274:24094-24099, 1999). However, Grb10 is not ubiquitinated by Nedd4 in cells. Here we show that in mouse embryo fibroblasts overexpressing Grb10 and the IGF-IR (p6/Grb10), there is a strong ligand-dependent increase in ubiquitination of the IGF-IR compared with that in parental cells (p6). This increased ubiquitination is associated with a shorter half-life and increased internalization of the IGF-IR. The IGF-IR is stabilized following treatment with both MG132 and chloroquine, indicating that both the proteasome and lysosomal pathways mediate degradation of the receptor. Ubiquitination of the IGF-IR likely occurs at the plasma membrane, prior to the formation of endocytic vesicles, as it is insensitive to dansylcadaverine, an inhibitor of early endosome formation in IGF-IR endocytosis. Grb10 coimmunoprecipitates with the IGF-IR and endogenous Nedd4 in p6/Grb10 cells, suggesting the presence of a Grb10/Nedd4/IGF-IR complex. Ubiquitination of the IGF-IR in p6/Grb10 cells is severely impaired by overexpression of a catalytically inactive Nedd4 mutant (Nedd4-CS), which also stabilizes the receptor. Likewise, overexpression of a Grb10 mutant lacking the Src homology 2 (SH2) domain impaired ubiquitination of the IGF-IR in parental p6 and p6/Grb10 cells, indicating that Grb10 binding to Nedd4 is critical for ubiquitination of the receptor. These results suggest a role for the Grb10/Nedd4 complex in regulating ubiquitination and stability of the IGF-IR, and they suggest that Grb10 serves as an adapter to form a bridge between Nedd4 and the IGF-IR. This is the first demonstration of regulation of stability of a tyrosine kinase receptor by the Nedd4 (HECT) family of E3 ligases.
Figures


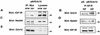

Similar articles
-
Grb10/Nedd4-mediated multiubiquitination of the insulin-like growth factor receptor regulates receptor internalization.J Cell Physiol. 2008 Aug;216(2):426-37. doi: 10.1002/jcp.21405. J Cell Physiol. 2008. PMID: 18286479
-
Nedd4 regulates ubiquitination and stability of the guanine-nucleotide exchange factor CNrasGEF.J Biol Chem. 2001 Dec 14;276(50):46995-7003. doi: 10.1074/jbc.M108373200. Epub 2001 Oct 11. J Biol Chem. 2001. PMID: 11598133
-
Grb10 prevents Nedd4-mediated vascular endothelial growth factor receptor-2 degradation.J Biol Chem. 2004 Jun 18;279(25):26754-61. doi: 10.1074/jbc.M311802200. Epub 2004 Apr 1. J Biol Chem. 2004. PMID: 15060076
-
Grb10 adapter protein as regulator of insulin-like growth factor receptor signaling.J Cell Physiol. 2003 Dec;197(3):307-11. doi: 10.1002/jcp.10363. J Cell Physiol. 2003. PMID: 14566960 Review.
-
Grb10 exceeding the boundaries of a common signaling adapter.Front Biosci. 2004 Jan 1;9:603-18. doi: 10.2741/1227. Front Biosci. 2004. PMID: 14766395 Review.
Cited by
-
FLT3 signals via the adapter protein Grb10 and overexpression of Grb10 leads to aberrant cell proliferation in acute myeloid leukemia.Mol Oncol. 2013 Jun;7(3):402-18. doi: 10.1016/j.molonc.2012.11.003. Epub 2012 Nov 29. Mol Oncol. 2013. PMID: 23246379 Free PMC article.
-
NEDD4: The founding member of a family of ubiquitin-protein ligases.Gene. 2015 Feb 25;557(2):113-22. doi: 10.1016/j.gene.2014.12.020. Epub 2014 Dec 17. Gene. 2015. PMID: 25527121 Free PMC article. Review.
-
Thiosemicarbazones and selected tyrosine kinase inhibitors synergize in pediatric solid tumors: NDRG1 upregulation and impaired prosurvival signaling in neuroblastoma cells.Front Pharmacol. 2022 Sep 7;13:976955. doi: 10.3389/fphar.2022.976955. eCollection 2022. Front Pharmacol. 2022. PMID: 36160437 Free PMC article.
-
Upregulation of the E3 ligase NEDD4-1 by oxidative stress degrades IGF-1 receptor protein in neurodegeneration.J Neurosci. 2012 Aug 8;32(32):10971-81. doi: 10.1523/JNEUROSCI.1836-12.2012. J Neurosci. 2012. PMID: 22875931 Free PMC article.
-
Lysine 63-linked polyubiquitination of the dopamine transporter requires WW3 and WW4 domains of Nedd4-2 and UBE2D ubiquitin-conjugating enzymes.J Biol Chem. 2010 Mar 5;285(10):7645-56. doi: 10.1074/jbc.M109.058990. Epub 2010 Jan 5. J Biol Chem. 2010. PMID: 20051513 Free PMC article.
References
-
- Aviel, S., G. Winberg, M. Massucci, and A. Ciechanover. 2000. Degradation of the Epstein-Barr virus latent membrane protein 1 (LMP1) by the ubiquitin-proteasome pathway. Targeting via ubiquitination of the N-terminal residue. J. Biol. Chem. 275:23491-23499. - PubMed
-
- Bai, R. Y., T. Jahn, S. Schrem, G. Munzert, K. M. Weidner, J. Y. Wang, and J. Duyster. 1998. The SH2-containing adapter protein GRB10 interacts with BCR-ABL. Oncogene 17:941-948. - PubMed
-
- Bereziat, V., A. Kasus-Jacobi, D. Perdereau, B. Cariou, J. Girard, and A. F. Burnol. 2002. Inhibition of insulin receptor catalytic activity by the molecular adapter Grb14. J. Biol. Chem. 277:4845-4852. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
