Ste11p, a high-mobility-group box DNA-binding protein, undergoes pheromone- and nutrient-regulated nuclear-cytoplasmic shuttling
- PMID: 12697825
- PMCID: PMC153199
- DOI: 10.1128/MCB.23.9.3253-3264.2003
Ste11p, a high-mobility-group box DNA-binding protein, undergoes pheromone- and nutrient-regulated nuclear-cytoplasmic shuttling
Abstract
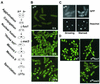
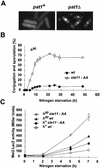
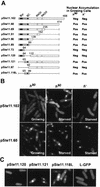
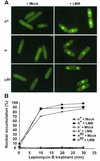
The high-mobility-group (HMG) box is a conserved DNA-binding domain found in a family of transcription factors that regulate growth and development. One family member, Ste11p, directs sexual differentiation of Schizosaccharomyces pombe by binding specific DNA sequences upstream of genes required for mating and meiosis. Here, we show that Ste11p is a shuttling protein. In growing cells, Ste11p is present in low levels and is pancellular. Mating pheromones and nutrient limitation trigger nuclear accumulation and increased expression of the transcription factor. Several mechanisms likely control Ste11p localization. First, the 14-3-3 protein, Rad24p, binds phosphorylated Ste11p and inhibits its nuclear accumulation. Second, the HMG domain of Ste11p contains a basic cluster nuclear localization signal. Finally, treatment of cells with leptomycin B, an exportin inhibitor, results in the nuclear accumulation of Ste11p. A Ste11p deletion mutation, DeltaC54, mimics the effects of leptomycin B. The C54 region contains no identifiable nuclear export signal but instead is required for biological activity and to stimulate Ste11p target gene expression. These results provide evidence that both nuclear import and export mechanisms operate to regulate cellular localization of an HMG box protein. In addition, they establish a paradigm for the potential role of pheromone/hormone-like polypeptides in cellular localization of this important class of developmental regulators.
Figures

Similar articles
-
Global roles of Ste11p, cell type, and pheromone in the control of gene expression during early sexual differentiation in fission yeast.Proc Natl Acad Sci U S A. 2006 Oct 17;103(42):15517-22. doi: 10.1073/pnas.0603403103. Epub 2006 Oct 9. Proc Natl Acad Sci U S A. 2006. PMID: 17032641 Free PMC article.
-
Crm1-mediated nuclear export of the Schizosaccharomyces pombe transcription factor Cuf1 during a shift from low to high copper concentrations.Eukaryot Cell. 2007 May;6(5):764-75. doi: 10.1128/EC.00002-07. Epub 2007 Mar 23. Eukaryot Cell. 2007. PMID: 17384198 Free PMC article.
-
Functional characterization of the interaction of Ste50p with Ste11p MAPKKK in Saccharomyces cerevisiae.Mol Biol Cell. 1999 Jul;10(7):2425-40. doi: 10.1091/mbc.10.7.2425. Mol Biol Cell. 1999. PMID: 10397774 Free PMC article.
-
[Control of gene expression in response to stress by a transcription factor Pap1].Tanpakushitsu Kakusan Koso. 1999 Nov;44(15 Suppl):2396-402. Tanpakushitsu Kakusan Koso. 1999. PMID: 10586689 Review. Japanese. No abstract available.
-
Regulation of sexual differentiation initiation in Schizosaccharomyces pombe.Biosci Biotechnol Biochem. 2024 Apr 22;88(5):475-492. doi: 10.1093/bbb/zbae019. Biosci Biotechnol Biochem. 2024. PMID: 38449372 Review.
Cited by
-
Cdc2p controls the forkhead transcription factor Fkh2p by phosphorylation during sexual differentiation in fission yeast.EMBO J. 2008 Jan 9;27(1):132-42. doi: 10.1038/sj.emboj.7601949. Epub 2007 Dec 6. EMBO J. 2008. PMID: 18059475 Free PMC article.
-
Sporulation: A response to starvation in the fission yeast Schizosaccharomyces pombe.Microbiologyopen. 2022 Jun;11(3):e1303. doi: 10.1002/mbo3.1303. Microbiologyopen. 2022. PMID: 35765188 Free PMC article. Review.
-
Multistep regulation of protein kinase A in its localization, phosphorylation and binding with a regulatory subunit in fission yeast.Curr Genet. 2011 Oct;57(5):353-65. doi: 10.1007/s00294-011-0354-2. Epub 2011 Aug 31. Curr Genet. 2011. PMID: 21879336
-
Transcriptome analysis implicates secondary metabolite production, redox reactions, and programmed cell death during allorecognition in Cryphonectria parasitica.G3 (Bethesda). 2021 Jan 18;11(1):jkaa021. doi: 10.1093/g3journal/jkaa021. G3 (Bethesda). 2021. PMID: 33561228 Free PMC article.
-
Cdk phosphorylation of the Ste11 transcription factor constrains differentiation-specific transcription to G1.Genes Dev. 2007 Feb 1;21(3):347-59. doi: 10.1101/gad.407107. Genes Dev. 2007. PMID: 17289922 Free PMC article.
References
-
- Alfa, C., P. Fantes, J. Hyams, M. McLeod, and E. Warbrick. 1993. Experiments with fission yeast. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
-
- Beach, D., L. Rodgers, and J. Gould. 1985. ran1+ controls the transition from mitotic division to meiosis in fission yeast. Curr. Genet. 10:297-311. - PubMed
-
- Bresch, C., G. Muller, and R. Egel. 1968. Genes involved in meiosis and sporulation of a yeast. Mol. Gen. Genet. 102:301-306. - PubMed
-
- Christophe, D., C. Christophe-Hobertus, and B. Pichon. 2000. Nuclear targeting of proteins: how many different signals? Cell Signal. 12:337-341. - PubMed
-
- Davey, J. 1998. Fusion of a fission yeast. Yeast 14:1529-1566. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
