Human rhinovirus type 2 is internalized by clathrin-mediated endocytosis
- PMID: 12692238
- PMCID: PMC153964
- DOI: 10.1128/jvi.77.9.5360-5369.2003
Human rhinovirus type 2 is internalized by clathrin-mediated endocytosis
Abstract
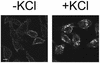
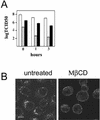
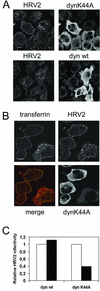
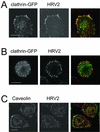
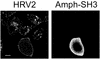
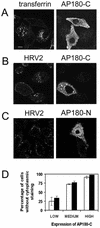
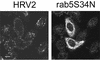
Using several approaches, we investigated the importance of clathrin-mediated endocytosis in the uptake of human rhinovirus serotype 2 (HRV2). By means of confocal immunofluorescence microscopy, we show that K(+) depletion strongly reduces HRV2 internalization. Viral uptake was also substantially reduced by extraction of cholesterol from the plasma membrane with methyl-beta-cyclodextrin, which can inhibit clathrin-mediated endocytosis. In accordance with these data, overexpression of dynamin K44A in HeLa cells prevented HRV2 internalization, as judged by confocal immunofluorescence microscopy, and strongly reduced infection. We also demonstrate that HRV2 bound to the surface of HeLa cells is localized in coated pits but not in caveolae. Finally, transient overexpression of the specific dominant-negative inhibitors of clathrin-mediated endocytosis, the SH3 domain of amphiphysin and the C-terminal domain of AP180, potently inhibited internalization of HRV2. Taken together, these results indicate that HRV2 uses clathrin-mediated endocytosis to infect cells.
Figures
Similar articles
-
Inhibition of clathrin-dependent endocytosis has multiple effects on human rhinovirus serotype 2 cell entry.J Biol Chem. 2001 Feb 9;276(6):3952-62. doi: 10.1074/jbc.M004722200. Epub 2000 Nov 9. J Biol Chem. 2001. PMID: 11073943
-
Entry of Classical Swine Fever Virus into PK-15 Cells via a pH-, Dynamin-, and Cholesterol-Dependent, Clathrin-Mediated Endocytic Pathway That Requires Rab5 and Rab7.J Virol. 2016 Sep 29;90(20):9194-208. doi: 10.1128/JVI.00688-16. Print 2016 Oct 15. J Virol. 2016. PMID: 27489278 Free PMC article.
-
Clathrin exchange during clathrin-mediated endocytosis.J Cell Biol. 2001 Oct 15;155(2):291-300. doi: 10.1083/jcb.200104085. Epub 2001 Oct 15. J Cell Biol. 2001. PMID: 11604424 Free PMC article.
-
Respiratory syncytial virus glycoproteins uptake occurs through clathrin-mediated endocytosis in a human epithelial cell line.Virol J. 2008 Oct 25;5:127. doi: 10.1186/1743-422X-5-127. Virol J. 2008. PMID: 18950517 Free PMC article. Review.
-
The amphiphysin family of proteins and their role in endocytosis at the synapse.Trends Neurosci. 1998 Aug;21(8):339-44. doi: 10.1016/s0166-2236(98)01264-8. Trends Neurosci. 1998. PMID: 9720601 Review.
Cited by
-
Interactions between virus proteins and host cell membranes during the viral life cycle.Int Rev Cytol. 2005;245:171-244. doi: 10.1016/S0074-7696(05)45006-8. Int Rev Cytol. 2005. PMID: 16125548 Free PMC article. Review.
-
Mechanisms of single-stranded phosphorothioate modified antisense oligonucleotide accumulation in hepatocytes.Nucleic Acids Res. 2011 Jun;39(11):4795-807. doi: 10.1093/nar/gkr089. Epub 2011 Feb 23. Nucleic Acids Res. 2011. PMID: 21345934 Free PMC article.
-
Recombinant VP4 of human rhinovirus induces permeability in model membranes.J Virol. 2008 Apr;82(8):4169-74. doi: 10.1128/JVI.01070-07. Epub 2008 Feb 6. J Virol. 2008. PMID: 18256154 Free PMC article.
-
Human rhinovirus-induced inflammatory responses are inhibited by phosphatidylserine containing liposomes.Mucosal Immunol. 2016 Sep;9(5):1303-16. doi: 10.1038/mi.2015.137. Epub 2016 Feb 24. Mucosal Immunol. 2016. PMID: 26906404 Free PMC article.
-
Human rhinovirus 14 enters rhabdomyosarcoma cells expressing icam-1 by a clathrin-, caveolin-, and flotillin-independent pathway.J Virol. 2010 Apr;84(8):3984-92. doi: 10.1128/JVI.01693-09. Epub 2010 Feb 3. J Virol. 2010. PMID: 20130060 Free PMC article.
References
-
- Basak, S., and H. Turner. 1992. Infectious entry pathway for canine parvovirus. Virology 186:368-376. - PubMed
-
- Bayer, N., D. Schober, M. Huttinger, D. Blaas, and R. Fuchs. 2001. Inhibition of clathrin-dependent endocytosis has multiple effects on human rhinovirus serotype 2 cell entry. J. Biol. Chem. 276:3952-3962. - PubMed
-
- Blake, K., and S. O'Connell. 1993. Virus culture, p. 81-122. In D. R. Harper (ed.), Virology labfax. Blackwell Scientific Publications, West Smithfield, London, United Kingdom.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

