Analysis of the mechanism by which the small-molecule CCR5 antagonists SCH-351125 and SCH-350581 inhibit human immunodeficiency virus type 1 entry
- PMID: 12692222
- PMCID: PMC153966
- DOI: 10.1128/jvi.77.9.5201-5208.2003
Analysis of the mechanism by which the small-molecule CCR5 antagonists SCH-351125 and SCH-350581 inhibit human immunodeficiency virus type 1 entry
Abstract
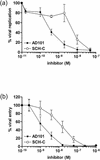
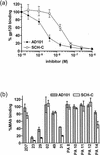
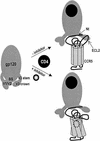
Human immunodeficiency virus type 1 (HIV-1) entry is mediated by the consecutive interaction of the envelope glycoprotein gp120 with CD4 and a coreceptor such as CCR5 or CXCR4. The CCR5 coreceptor is used by the most commonly transmitted HIV-1 strains that often persist throughout the course of infection. Compounds targeting CCR5-mediated entry are a novel class of drugs being developed to treat HIV-1 infection. In this study, we have identified the mechanism of action of two inhibitors of CCR5 function, SCH-350581 (AD101) and SCH-351125 (SCH-C). AD101 is more potent than SCH-C at inhibiting HIV-1 replication in primary lymphocytes, as well as viral entry and gp120 binding to cell lines. Both molecules also block the binding of several anti-CCR5 monoclonal antibodies that recognize epitopes in the second extracellular loop of CCR5. Alanine mutagenesis of the transmembrane domain of CCR5 suggests that AD101 and SCH-C bind to overlapping but nonidentical sites within a putative ligand-binding cavity formed by transmembrane helices 1, 2, 3, and 7. We propose that the binding of small molecules to the transmembrane domain of CCR5 may disrupt the conformation of its extracellular domain, thereby inhibiting ligand binding to CCR5.
Figures

Similar articles
-
The differential sensitivity of human and rhesus macaque CCR5 to small-molecule inhibitors of human immunodeficiency virus type 1 entry is explained by a single amino acid difference and suggests a mechanism of action for these inhibitors.J Virol. 2004 Apr;78(8):4134-44. doi: 10.1128/jvi.78.8.4134-4144.2004. J Virol. 2004. PMID: 15047829 Free PMC article.
-
The role of the N-terminal segment of CCR5 in HIV-1 Env-mediated membrane fusion and the mechanism of virus adaptation to CCR5 lacking this segment.Retrovirology. 2007 Aug 8;4:55. doi: 10.1186/1742-4690-4-55. Retrovirology. 2007. PMID: 17686153 Free PMC article.
-
A binding pocket for a small molecule inhibitor of HIV-1 entry within the transmembrane helices of CCR5.Proc Natl Acad Sci U S A. 2000 May 9;97(10):5639-44. doi: 10.1073/pnas.090576697. Proc Natl Acad Sci U S A. 2000. PMID: 10779565 Free PMC article.
-
[Chemokine receptors and its importance in the replication cycle of human immunodeficiency virus: clinical and therapeutic implications].Acta Med Port. 2008 Sep-Oct;21(5):497-504. Epub 2009 Jan 16. Acta Med Port. 2008. PMID: 19187693 Review. Portuguese.
-
Small-molecule antagonists of CCR5 and CXCR4: a promising new class of anti-HIV-1 drugs.Curr Pharm Des. 2004;10(17):2041-62. doi: 10.2174/1381612043384312. Curr Pharm Des. 2004. PMID: 15279544 Review.
Cited by
-
CCR5 interactions with the variable 3 loop of gp120.J Mol Model. 2007 Jan;13(1):29-41. doi: 10.1007/s00894-006-0117-z. Epub 2006 May 24. J Mol Model. 2007. PMID: 16721558
-
Small molecules blocking the entry of severe acute respiratory syndrome coronavirus into host cells.J Virol. 2004 Oct;78(20):11334-9. doi: 10.1128/JVI.78.20.11334-11339.2004. J Virol. 2004. PMID: 15452254 Free PMC article.
-
Variants of CCR5, which are permissive for HIV-1 infection, show distinct functional responses to CCL3, CCL4 and CCL5.Genes Immun. 2005 Oct;6(7):609-19. doi: 10.1038/sj.gene.6364247. Genes Immun. 2005. PMID: 16015368 Free PMC article.
-
Structure-function analysis of human immunodeficiency virus type 1 gp120 amino acid mutations associated with resistance to the CCR5 coreceptor antagonist vicriviroc.J Virol. 2009 Dec;83(23):12151-63. doi: 10.1128/JVI.01351-09. Epub 2009 Sep 23. J Virol. 2009. PMID: 19776131 Free PMC article.
-
Binding modes of CCR5-targetting HIV entry inhibitors: partial and full antagonists.J Mol Graph Model. 2008 Jun;26(8):1287-95. doi: 10.1016/j.jmgm.2007.12.003. Epub 2007 Dec 17. J Mol Graph Model. 2008. PMID: 18249144 Free PMC article.
References
-
- Baba, M., O. Nishimura, N. Kanzaki, M. Okamoto, H. Sawada, Y. Iizawa, M. Shiraishi, Y. Aramaki, K. Okonogi, Y. Ogawa, K. Meguro, and M. Fujino. 1999. A small-molecule, nonpeptide CCR5 antagonist with highly potent and selective anti-HIV-1 activity. Proc. Natl. Acad. Sci. USA 96:5698-5703. - PMC - PubMed
-
- Berger, E. A., P. M. Murphy, and J. M. Farber. 1999. Chemokine receptors as HIV-1 coreceptors: roles in viral entry, tropism, and disease. Annu. Rev. Immunol. 17:657-700. - PubMed
-
- Cocchi, F., A. L. DeVico, A. Garzino-Demo, S. K. Arya, R. C. Gallo, and P. Lusso. 1995. Identification of RANTES, MIP-1 alpha, and MIP-1 beta as the major HIV-suppressive factors produced by CD8+ T cells. Science 270:1811-1815. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

