Signal transduction by mechanical strain in chondrocytes
- PMID: 12690261
- PMCID: PMC4947461
- DOI: 10.1097/01.mco.0000068964.34812.2b
Signal transduction by mechanical strain in chondrocytes
Abstract
Purpose of review: Exercise and passive motion exert reparative effects on inflamed joints, whereas excessive mechanical forces initiate cartilage destruction as observed in osteoarthritis. However, the intracellular mechanisms that convert mechanical signals into biochemical events responsible for cartilage destruction and repair remain paradoxical. This review summarizes how signals generated by mechanical stress may initiate repair or destruction of cartilage.
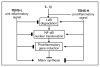
Recent findings: Mechanical strain of low magnitude inhibits inflammation by suppressing IL-1beta and TNF-alpha-induced transcription of multiple proinflammatory mediators involved in cartilage degradation. This also results in the upregulation of proteoglycan and collagen synthesis that is drastically inhibited in inflamed joints. On the contrary, mechanical strain of high magnitude is proinflammatory and initiates cartilage destruction while inhibiting matrix synthesis. Investigations reveal that mechanical signals exploit nuclear factor-kappa B as a common pathway for transcriptional inhibition/activation of proinflammatory genes to control catabolic processes in chondrocytes. Mechanical strain of low magnitude prevents nuclear translocation of nuclear factor kappa B, resulting in the suppression of proinflammatory gene expression, whereas mechanical strain of high magnitude induces transactivation of nuclear factor kappa B, and thus proinflammatory gene induction.
Summary: The beneficial effects of physiological levels of mechanical signals or exercise may be explained by their ability to suppress the signal transduction pathways of proinflammatory/catabolic mediators, while stimulating anabolic pathways. Whether these anabolic signals are a consequence of the inhibition of nuclear factor kappa B or are mediated via distinct anabolic pathways is yet to be elucidated.
Figures
Similar articles
-
Role of NF-kappaB transcription factors in antiinflammatory and proinflammatory actions of mechanical signals.Arthritis Rheum. 2004 Nov;50(11):3541-8. doi: 10.1002/art.20601. Arthritis Rheum. 2004. PMID: 15529376 Free PMC article.
-
Tumor necrosis factor alpha-dependent proinflammatory gene induction is inhibited by cyclic tensile strain in articular chondrocytes in vitro.Arthritis Rheum. 2001 Oct;44(10):2311-9. doi: 10.1002/1529-0131(200110)44:10<2311::aid-art393>3.0.co;2-q. Arthritis Rheum. 2001. PMID: 11665971 Free PMC article.
-
Modulation of TGF-beta signaling by proinflammatory cytokines in articular chondrocytes.Osteoarthritis Cartilage. 2007 Dec;15(12):1367-77. doi: 10.1016/j.joca.2007.04.011. Epub 2007 Jun 29. Osteoarthritis Cartilage. 2007. PMID: 17604656 Free PMC article.
-
Regulation of chondrocytic gene expression by biomechanical signals.Crit Rev Eukaryot Gene Expr. 2008;18(2):139-50. doi: 10.1615/critreveukargeneexpr.v18.i2.30. Crit Rev Eukaryot Gene Expr. 2008. PMID: 18304028 Free PMC article. Review.
-
Effects of shear stress on articular chondrocyte metabolism.Biorheology. 2000;37(1-2):95-107. Biorheology. 2000. PMID: 10912182 Review.
Cited by
-
A synoptic literature review of animal models for investigating the biomechanics of knee osteoarthritis.Front Bioeng Biotechnol. 2024 Jul 25;12:1408015. doi: 10.3389/fbioe.2024.1408015. eCollection 2024. Front Bioeng Biotechnol. 2024. PMID: 39132255 Free PMC article. Review.
-
Roles of inflammatory and anabolic cytokines in cartilage metabolism: signals and multiple effectors converge upon MMP-13 regulation in osteoarthritis.Eur Cell Mater. 2011 Feb 24;21:202-20. doi: 10.22203/ecm.v021a16. Eur Cell Mater. 2011. PMID: 21351054 Free PMC article.
-
Intermittent Cyclic Mechanical Tension Promotes Degeneration of Endplate Cartilage via the Nuclear Factor-κB Signaling Pathway: an in Vivo Study.Orthop Surg. 2016 Aug;8(3):393-9. doi: 10.1111/os.12260. Orthop Surg. 2016. PMID: 27627724 Free PMC article.
-
Silencing of circ_0000205 mitigates interleukin-1β-induced apoptosis and extracellular matrix degradation in chondrocytes via targeting miR-766-3p/ADAMTS5 axis.Innate Immun. 2022 Feb;28(2):79-90. doi: 10.1177/17534259221077078. Innate Immun. 2022. PMID: 35484121 Free PMC article.
-
Dynamic compression alters NFkappaB activation and IkappaB-alpha expression in IL-1beta-stimulated chondrocyte/agarose constructs.Inflamm Res. 2010 Jan;59(1):41-52. doi: 10.1007/s00011-009-0068-9. Epub 2009 Aug 8. Inflamm Res. 2010. PMID: 19669392
References
-
- Frenkel SR, Di Cesare PE. Degradation and repair of articular cartilage. Front Biosci. 1999;15:D671–D685. - PubMed
-
- Salter DM, Millward-Sadler SJ, Nuki G, Wright MO. Integrin-interleukin-4 mechanotransduction pathways in human chondrocytes. Clin Orthop. 2001;391(Suppl):S49–S60. - PubMed
-
- Loeser RF. Integrins and cell signaling in chondrocytes. Biorheology. 2002;39:119–124. This article provides a comprehensive review on integrins, MAP kinases, and focal adhesion kinases involved in mechanical signaling. - PubMed
-
- Goldring MB. The role of cytokines as inflammatory mediators in osteoarthritis: lessons from animal models. Connective Tiss Res. 1999;40:1–11. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

