Accumulation of cytoplasmic dynein and dynactin at microtubule plus ends in Aspergillus nidulans is kinesin dependent
- PMID: 12686603
- PMCID: PMC153116
- DOI: 10.1091/mbc.e02-08-0516
Accumulation of cytoplasmic dynein and dynactin at microtubule plus ends in Aspergillus nidulans is kinesin dependent
Abstract
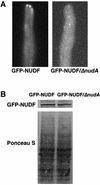
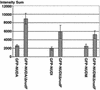
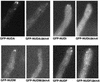
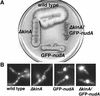
The mechanism(s) by which microtubule plus-end tracking proteins are targeted is unknown. In the filamentous fungus Aspergillus nidulans, both cytoplasmic dynein and NUDF, the homolog of the LIS1 protein, localize to microtubule plus ends as comet-like structures. Herein, we show that NUDM, the p150 subunit of dynactin, also forms dynamic comet-like structures at microtubule plus ends. By examining proteins tagged with green fluorescent protein in different loss-of-function mutants, we demonstrate that dynactin and cytoplasmic dynein require each other for microtubule plus-end accumulation, and the presence of cytoplasmic dynein is also important for NUDF's plus-end accumulation. Interestingly, deletion of NUDF increases the overall accumulation of dynein and dynactin at plus ends, suggesting that NUDF may facilitate minus-end-directed dynein movement. Finally, we demonstrate that a conventional kinesin, KINA, is required for the microtubule plus-end accumulation of cytoplasmic dynein and dynactin, but not of NUDF.
Figures




Similar articles
-
The Aspergillus cytoplasmic dynein heavy chain and NUDF localize to microtubule ends and affect microtubule dynamics.Curr Biol. 2001 May 1;11(9):719-24. doi: 10.1016/s0960-9822(01)00200-7. Curr Biol. 2001. PMID: 11369237
-
CLIP-170 homologue and NUDE play overlapping roles in NUDF localization in Aspergillus nidulans.Mol Biol Cell. 2006 Apr;17(4):2021-34. doi: 10.1091/mbc.e05-11-1084. Epub 2006 Feb 8. Mol Biol Cell. 2006. PMID: 16467375 Free PMC article.
-
In vivo roles of the basic domain of dynactin p150 in microtubule plus-end tracking and dynein function.Traffic. 2012 Mar;13(3):375-87. doi: 10.1111/j.1600-0854.2011.01312.x. Epub 2011 Dec 18. Traffic. 2012. PMID: 22106867 Free PMC article.
-
Cytoplasmic dynein and early endosome transport.Cell Mol Life Sci. 2015 Sep;72(17):3267-80. doi: 10.1007/s00018-015-1926-y. Epub 2015 May 23. Cell Mol Life Sci. 2015. PMID: 26001903 Free PMC article. Review.
-
Cytoplasmic dynein and dynactin in cell division and intracellular transport.Curr Opin Cell Biol. 1999 Feb;11(1):45-53. doi: 10.1016/s0955-0674(99)80006-4. Curr Opin Cell Biol. 1999. PMID: 10047518 Review.
Cited by
-
The myosin motor domain of fungal chitin synthase V is dispensable for vesicle motility but required for virulence of the maize pathogen Ustilago maydis.Plant Cell. 2010 Jul;22(7):2476-94. doi: 10.1105/tpc.110.075028. Epub 2010 Jul 27. Plant Cell. 2010. PMID: 20663961 Free PMC article.
-
A +TIP for a smooth trip.J Cell Biol. 2006 Feb 27;172(5):651-4. doi: 10.1083/jcb.200511081. Epub 2006 Feb 21. J Cell Biol. 2006. PMID: 16492810 Free PMC article. Review.
-
Cytoplasmic dynein1 intermediate-chain2 regulates cellular trafficking and physiopathological development in Magnaporthe oryzae.iScience. 2023 Feb 10;26(2):106050. doi: 10.1016/j.isci.2023.106050. eCollection 2023 Feb 17. iScience. 2023. PMID: 36866040 Free PMC article.
-
Dynein-dependent motility of microtubules and nucleation sites supports polarization of the tubulin array in the fungus Ustilago maydis.Mol Biol Cell. 2006 Jul;17(7):3242-53. doi: 10.1091/mbc.e05-12-1118. Epub 2006 May 3. Mol Biol Cell. 2006. PMID: 16672380 Free PMC article.
-
p25 of the dynactin complex plays a dual role in cargo binding and dynactin regulation.J Biol Chem. 2018 Oct 5;293(40):15606-15619. doi: 10.1074/jbc.RA118.004000. Epub 2018 Aug 24. J Biol Chem. 2018. PMID: 30143531 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

