HIV reproducibly establishes a latent infection after acute infection of T cells in vitro
- PMID: 12682019
- PMCID: PMC154479
- DOI: 10.1093/emboj/cdg188
HIV reproducibly establishes a latent infection after acute infection of T cells in vitro
Abstract
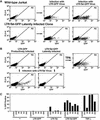
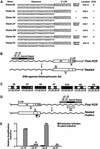
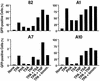
The presence of latent reservoirs has prevented the eradication of human immunodeficiency virus (HIV) from infected patients successfully treated with anti-retroviral therapy. The mechanism of postintegration latency is poorly understood, partly because of the lack of an in vitro model. We have used an HIV retroviral vector or a full-length HIV genome expressing green fluorescent protein to infect a T lymphocyte cell line in vitro and highly enrich for latently infected cells. HIV latency occurred reproducibly, albeit with low frequency, during an acute infection. Clonal cell lines derived from latent populations showed no detectable basal expression, but could be transcriptionally activated after treatment with phorbol esters or tumor necrosis factor alpha. Direct sequencing of integration sites demonstrated that latent clones frequently contain HIV integrated in or close to alphoid repeat elements in heterochromatin. This is in contrast to a productive infection where integration in or near heterochromatin is disfavored. These observations demonstrate that HIV can reproducibly establish a latent infection as a consequence of integration in or near heterochromatin.
Figures


Similar articles
-
HIV Provirus Stably Reproduces Parental Latent and Induced Transcription Phenotypes Regardless of the Chromosomal Integration Site.J Virol. 2016 May 12;90(11):5302-14. doi: 10.1128/JVI.02842-15. Print 2016 Jun 1. J Virol. 2016. PMID: 26984732 Free PMC article.
-
Transcriptional suppression of in vitro-integrated human immunodeficiency virus type 1 does not correlate with proviral DNA methylation.J Virol. 2003 Apr;77(7):4025-32. doi: 10.1128/jvi.77.7.4025-4032.2003. J Virol. 2003. PMID: 12634362 Free PMC article.
-
Gradual shutdown of virus production resulting in latency is the norm during the chronic phase of human immunodeficiency virus replication and differential rates and mechanisms of shutdown are determined by viral sequences.Virology. 1996 Nov 1;225(1):196-212. doi: 10.1006/viro.1996.0588. Virology. 1996. PMID: 8918547
-
Experimental approaches to the study of HIV-1 latency.Nat Rev Microbiol. 2007 Feb;5(2):95-106. doi: 10.1038/nrmicro1580. Nat Rev Microbiol. 2007. PMID: 17224919 Review.
-
Reservoirs for HIV-1: mechanisms for viral persistence in the presence of antiviral immune responses and antiretroviral therapy.Annu Rev Immunol. 2000;18:665-708. doi: 10.1146/annurev.immunol.18.1.665. Annu Rev Immunol. 2000. PMID: 10837072 Review.
Cited by
-
Optimization of a lentivirus-mediated gene therapy targeting HIV-1 RNA to eliminate HIV-1-infected cells.Mol Ther Nucleic Acids. 2024 Sep 16;35(4):102341. doi: 10.1016/j.omtn.2024.102341. eCollection 2024 Dec 10. Mol Ther Nucleic Acids. 2024. PMID: 39434850 Free PMC article.
-
Discovery of new acetamide derivatives of 5-indole-1,3,4-oxadiazol-2-thiol as inhibitors of HIV-1 Tat-mediated viral transcription.Antimicrob Agents Chemother. 2024 Oct 8;68(10):e0064324. doi: 10.1128/aac.00643-24. Epub 2024 Sep 4. Antimicrob Agents Chemother. 2024. PMID: 39230310 Free PMC article.
-
The HIV-1 Tat Protein Is Monomethylated at Lysine 71 by the Lysine Methyltransferase KMT7.J Biol Chem. 2016 Jul 29;291(31):16240-8. doi: 10.1074/jbc.M116.735415. Epub 2016 May 27. J Biol Chem. 2016. PMID: 27235396 Free PMC article.
-
Reactivating latent HIV with PKC agonists induces resistance to apoptosis and is associated with phosphorylation and activation of BCL2.PLoS Pathog. 2020 Oct 19;16(10):e1008906. doi: 10.1371/journal.ppat.1008906. eCollection 2020 Oct. PLoS Pathog. 2020. PMID: 33075109 Free PMC article.
-
HIV-1 Transcription but Not Intact Provirus Levels are Associated With Systemic Inflammation.J Infect Dis. 2021 Jun 4;223(11):1934-1942. doi: 10.1093/infdis/jiaa657. J Infect Dis. 2021. PMID: 33075121 Free PMC article.
References
-
- Antoni B.A., Rabson,A.B., Kinter,A., Bodkin,M. and Poli,G. (1994) NF-κB-dependent and -independent pathways of HIV activation in a chronically infected T cell line. Virology, 202, 684–694. - PubMed
-
- Butera S.T. (2000) Therapeutic targeting of human immunodeficiency virus type-1 latency: Current clinical realities and future scientific possibilities. Antiviral Res., 48, 143–176. - PubMed
-
- Butler S.L., Hansen,M.S. and Bushman,F.D. (2001) A quantitative assay for HIV DNA integration in vivo. Nat. Med., 7, 631–634. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

