Phosphorylation of serine 303 is a prerequisite for the stress-inducible SUMO modification of heat shock factor 1
- PMID: 12665592
- PMCID: PMC152542
- DOI: 10.1128/MCB.23.8.2953-2968.2003
Phosphorylation of serine 303 is a prerequisite for the stress-inducible SUMO modification of heat shock factor 1
Abstract
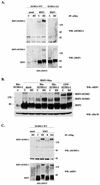
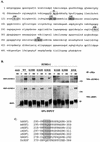
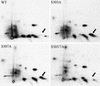
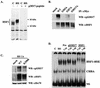
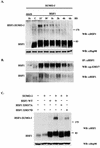
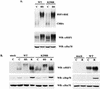
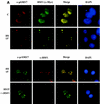
The heat shock response, which is accompanied by a rapid and robust upregulation of heat shock proteins (Hsps), is a highly conserved protection mechanism against protein-damaging stress. Hsp induction is mainly regulated at transcriptional level by stress-inducible heat shock factor 1 (HSF1). Upon activation, HSF1 trimerizes, binds to DNA, concentrates in the nuclear stress granules, and undergoes a marked multisite phosphorylation, which correlates with its transcriptional activity. In this study, we show that HSF1 is modified by SUMO-1 and SUMO-2 in a stress-inducible manner. Sumoylation is rapidly and transiently enhanced on lysine 298, located in the regulatory domain of HSF1, adjacent to several critical phosphorylation sites. Sumoylation analyses of HSF1 phosphorylation site mutants reveal that specifically the phosphorylation-deficient S303 mutant remains devoid of SUMO modification in vivo and the mutant mimicking phosphorylation of S303 promotes HSF1 sumoylation in vitro, indicating that S303 phosphorylation is required for K298 sumoylation. This finding is further supported by phosphopeptide mapping and analysis with S303/7 phosphospecific antibodies, which demonstrate that serine 303 is a target for strong heat-inducible phosphorylation, corresponding to the inducible HSF1 sumoylation. A transient phosphorylation-dependent colocalization of HSF1 and SUMO-1 in nuclear stress granules provides evidence for a strictly regulated subnuclear interplay between HSF1 and SUMO.
Figures




Similar articles
-
Insights into the regulation of heat shock transcription factor 1 SUMO-1 modification.Biochem Biophys Res Commun. 2003 Mar 28;303(1):196-200. doi: 10.1016/s0006-291x(03)00312-7. Biochem Biophys Res Commun. 2003. PMID: 12646186
-
Heat shock protein 27 is involved in SUMO-2/3 modification of heat shock factor 1 and thereby modulates the transcription factor activity.Oncogene. 2009 Sep 17;28(37):3332-44. doi: 10.1038/onc.2009.188. Epub 2009 Jul 13. Oncogene. 2009. PMID: 19597476
-
Heat shock transcription factor 1 is SUMOylated in the activated trimeric state.J Biol Chem. 2021 Jan-Jun;296:100324. doi: 10.1016/j.jbc.2021.100324. Epub 2021 Jan 23. J Biol Chem. 2021. PMID: 33493517 Free PMC article.
-
Regulation of heat shock transcription factor 1 by stress-induced SUMO-1 modification.J Biol Chem. 2001 Oct 26;276(43):40263-7. doi: 10.1074/jbc.M104714200. Epub 2001 Aug 20. J Biol Chem. 2001. PMID: 11514557
-
Deciphering human heat shock transcription factor 1 regulation via post-translational modification in yeast.PLoS One. 2011 Jan 6;6(1):e15976. doi: 10.1371/journal.pone.0015976. PLoS One. 2011. PMID: 21253609 Free PMC article.
Cited by
-
Protein inhibitor of activated STAT3 (PIAS3) protein promotes SUMOylation and nuclear sequestration of the intracellular domain of ErbB4 protein.J Biol Chem. 2012 Jun 29;287(27):23216-26. doi: 10.1074/jbc.M111.335927. Epub 2012 May 14. J Biol Chem. 2012. PMID: 22584572 Free PMC article.
-
HSF1 and Its Role in Huntington's Disease Pathology.Adv Exp Med Biol. 2023;1410:35-95. doi: 10.1007/5584_2022_742. Adv Exp Med Biol. 2023. PMID: 36396925 Review.
-
SUMO-specific protease 1 is critical for early lymphoid development through regulation of STAT5 activation.Mol Cell. 2012 Jan 27;45(2):210-21. doi: 10.1016/j.molcel.2011.12.026. Mol Cell. 2012. PMID: 22284677 Free PMC article.
-
SUMO conjugation in plants.Planta. 2004 Nov;220(1):1-8. doi: 10.1007/s00425-004-1370-y. Epub 2004 Sep 23. Planta. 2004. PMID: 15449058 Review.
-
Proteins of the PIAS family enhance the sumoylation of the papillomavirus E1 protein.Virology. 2005 Jan 5;331(1):190-203. doi: 10.1016/j.virol.2004.10.025. Virology. 2005. PMID: 15582666 Free PMC article.
References
-
- Appella, E., and C. W. Anderson. 2001. Post-translational modifications and activation of p53 by genotoxic stresses. Eur. J. Biochem. 268:2764-2772. - PubMed
-
- Ben-Neriah, Y. 2002. Regulatory functions of ubiquitination in the immune system. Nat. Immunol. 3:20-26. - PubMed
-
- Bies, J., J. Markus, and L. Wolff. 2002. Covalent attachment of the SUMO-1 protein to the negative regulatory domain of the c-Myb transcription factor modifies its stability and transactivation capacity. J. Biol. Chem. 277:8999-9009. - PubMed
-
- Chu, B., F. Soncin, B. D. Price, M. A. Stevenson, and S. K. Calderwood. 1996. Sequential phosphorylation by mitogen-activated protein kinase and glycogen synthase kinase 3 represses transcriptional activation by heat shock factor-1. J. Biol. Chem. 271:30847-30857. - PubMed
-
- Chu, B., R. Zhong, F. Soncin, M. A. Stevenson, and S. K. Calderwood. 1998. Transcriptional activity of heat shock factor 1 at 37°C is repressed through phosphorylation on two distinct serine residues by glycogen synthase kinase 3 and protein kinases Cα and Cζ. J. Biol. Chem. 273:18640-18646. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
