Constitutive inositol phosphate formation in cytomegalovirus-infected human fibroblasts is due to expression of the chemokine receptor homologue pUS28
- PMID: 12663756
- PMCID: PMC152109
- DOI: 10.1128/jvi.77.8.4489-4501.2003
Constitutive inositol phosphate formation in cytomegalovirus-infected human fibroblasts is due to expression of the chemokine receptor homologue pUS28
Abstract
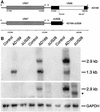
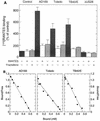
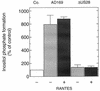
An open reading frame (ORF), US28, with homology to mammalian chemokine receptors has been identified in the genome of human cytomegalovirus (HCMV). Its protein product, pUS28, has been shown to bind several human CC chemokines, including RANTES, MCP-1, and MIP-1 alpha, and the CX(3)C chemokine fractalkine with high affinity. Addition of CC chemokines to cells expressing pUS28 was reported to cause a pertussis toxin-sensitive increase in the concentration of cytosolic free Ca(2+). Recently, pUS28 was shown to mediate constitutive, ligand-independent, and pertussis toxin-insensitive activation of phospholipase C via G(q/11)-dependent signaling pathways in transiently transfected COS-7 cells. Since these findings are not easily reconciled with the former observations, we analyzed the role of pUS28 in mediating CC chemokine activation of pertussis toxin-sensitive G proteins in cell membranes and phospholipase C in intact cells. The transmembrane signaling functions of pUS28 were studied in HCMV-infected cells rather than in cDNA-transfected cells. Since DNA sequence analysis of ORF US28 of different laboratory and clinical strains had revealed amino acid sequence differences in the amino-terminal portion of pUS28, we compared two laboratory HCMV strains, AD169 and Toledo, and one clinical strain, TB40/E. The results showed that infection of human fibroblasts with all three HCMV strains led to a vigorous, constitutively enhanced formation of inositol phosphates which was insensitive to pertussis toxin. This effect was critically dependent on the presence of the US28 ORF in the HCMV genome but was independent of the amino acid sequence divergence of the three HCMV strains investigated. The constitutive activity of pUS28 is not explained by expression of pUS28 at high density in HCMV-infected cells. The pUS28 ligands RANTES and MCP-1 failed to stimulate binding of guanosine 5'-O-(3-[(35)S]thiotriphosphate to membranes of HCMV-infected cells and did not enhance constitutive activation of phospholipase C in intact HCMV-infected cells. These findings raise the possibility that the effects of CC chemokines and pertussis toxin on G protein-mediated transmembrane signaling previously observed in HCMV-infected cells are either independent of or not directly mediated by the protein product of ORF US28.
Figures
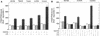
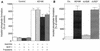


Similar articles
-
Chemokine sequestration by viral chemoreceptors as a novel viral escape strategy: withdrawal of chemokines from the environment of cytomegalovirus-infected cells.J Exp Med. 1998 Sep 7;188(5):855-66. doi: 10.1084/jem.188.5.855. J Exp Med. 1998. PMID: 9730887 Free PMC article.
-
Constitutive serum response factor activation by the viral chemokine receptor homologue pUS28 is differentially regulated by Galpha(q/11) and Galpha(16).Cell Signal. 2008 Aug;20(8):1528-37. doi: 10.1016/j.cellsig.2008.04.010. Epub 2008 Apr 24. Cell Signal. 2008. PMID: 18534820
-
Functional analysis of human cytomegalovirus pUS28 mutants in infected cells.J Gen Virol. 2008 Jan;89(Pt 1):97-105. doi: 10.1099/vir.0.83226-0. J Gen Virol. 2008. PMID: 18089733 Free PMC article.
-
Human Cytomegalovirus US28: a functionally selective chemokine binding receptor.Infect Disord Drug Targets. 2009 Nov;9(5):548-56. doi: 10.2174/187152609789105696. Infect Disord Drug Targets. 2009. PMID: 19594424 Free PMC article. Review.
-
US28: HCMV's Swiss Army Knife.Viruses. 2018 Aug 20;10(8):445. doi: 10.3390/v10080445. Viruses. 2018. PMID: 30127279 Free PMC article. Review.
Cited by
-
MLKL Requires the Inositol Phosphate Code to Execute Necroptosis.Mol Cell. 2018 Jun 7;70(5):936-948.e7. doi: 10.1016/j.molcel.2018.05.010. Epub 2018 Jun 7. Mol Cell. 2018. PMID: 29883610 Free PMC article.
-
Caspase-8-dependent control of NK- and T cell responses during cytomegalovirus infection.Med Microbiol Immunol. 2019 Aug;208(3-4):555-571. doi: 10.1007/s00430-019-00616-7. Epub 2019 May 16. Med Microbiol Immunol. 2019. PMID: 31098689 Review.
-
The constitutive activity of the viral-encoded G protein-coupled receptor US28 supports a complex signalling network contributing to cancer development.Biochem Soc Trans. 2020 Aug 28;48(4):1493-1504. doi: 10.1042/BST20190988. Biochem Soc Trans. 2020. PMID: 32779712 Free PMC article. Review.
-
The chemokine receptor homologue encoded by US27 of human cytomegalovirus is heavily glycosylated and is present in infected human foreskin fibroblasts and enveloped virus particles.Virus Res. 2007 Jan;123(1):57-71. doi: 10.1016/j.virusres.2006.08.003. Epub 2006 Sep 8. Virus Res. 2007. PMID: 16963142 Free PMC article.
-
Human cytomegalovirus encoded homologs of cytokines, chemokines and their receptors: roles in immunomodulation.Viruses. 2012 Oct 25;4(11):2448-70. doi: 10.3390/v4112448. Viruses. 2012. PMID: 23202490 Free PMC article. Review.
References
-
- Albrecht, T., M. Nachtigal, S. C. St Jeor, and F. Rapp. 1976. Induction of cellular DNA synthesis and increased mitotic activity in Syrian hamster embryo cells abortively infected with human cytomegalovirus. J. Gen. Virol. 30:167-177. - PubMed
-
- Albrecht, T., M. P. Fons, I. Boldogh, S. AbuBakar, C. Z. Deng, and D. Millinoff. 1991. Metabolic and cellular effects of human cytomegalovirus infection. Transplant. Proc. 23(Suppl. 3):48-55. - PubMed
-
- Althoefer, H., P. Eversole-Cire, and M. I. Simon. 1997. Constitutively active Gαq and Gα13 trigger apoptosis through different pathways. J. Biol. Chem. 272:24380-24386. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

