Expression studies of gibberellin oxidases in developing pumpkin seeds
- PMID: 12644672
- PMCID: PMC166882
- DOI: 10.1104/pp.015206
Expression studies of gibberellin oxidases in developing pumpkin seeds
Abstract
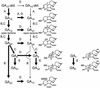
Two cDNA clones, 3-ox and 2-ox, have been isolated from developing pumpkin (Cucurbita maxima) embryos that show significant amino acid homology to gibberellin (GA) 3-oxidases and 2-oxidases, respectively. Recombinant fusion protein of clone 3-ox converted GA(12)-aldehyde, GA(12), GA(15), GA(24), GA(25), and GA(9) to GA(14)-aldehyde, GA(14), GA(37), GA(36), GA(13), and GA(4), respectively. Recombinant 2-ox protein oxidized GA(9), GA(4), and GA(1) to GA(51), GA(34), and GA(8), respectively. Previously cloned GA 7-oxidase revealed additional 3beta-hydroxylation activity of GA(12). Transcripts of this gene were identified in endosperm and embryo of the developing seed by quantitative reverse transcriptase-polymerase chain reaction and localized in protoderm, root apical meristem, and quiescent center by in situ hybridization. mRNA of the previously cloned GA 20-oxidase from pumpkin seeds was localized in endosperm and in tissues of protoderm, ground meristem, and cotyledons of the embryo. However, transcripts of the recently cloned GA 20-oxidase from pumpkin seedlings were found all over the embryo, and in tissues of the inner seed coat at the micropylar end. Previously cloned GA 2beta,3beta-hydroxylase mRNA molecules were specifically identified in endosperm tissue. Finally, mRNA molecules of the 3-ox and 2-ox genes were found in the embryo only. 3-ox transcripts were localized in tissues of cotyledons, protoderm, and inner cell layers of the root apical meristem, and 2-ox transcripts were found in all tissues of the embryo except the root tips. These results indicate tissue-specific GA-biosynthetic pathways operating within the developing seed.
Figures

Similar articles
-
Expression of two genes encoding gibberellin 2- and 3-oxidases in developing seeds of Phaseolus coccineus.Plant Cell Physiol. 2005 Jul;46(7):1116-24. doi: 10.1093/pcp/pci124. Epub 2005 May 13. Plant Cell Physiol. 2005. PMID: 15894806
-
Cloning and expression of a gibberellin 2 beta,3 beta-hydroxylase cDNA from pumpkin endosperm.Plant Cell. 1997 Aug;9(8):1459-67. doi: 10.1105/tpc.9.8.1459. Plant Cell. 1997. PMID: 9286114 Free PMC article.
-
Gibberellin biosynthesis in developing pumpkin seedlings.Plant Physiol. 2005 Sep;139(1):213-23. doi: 10.1104/pp.105.064162. Epub 2005 Aug 26. Plant Physiol. 2005. PMID: 16126862 Free PMC article.
-
Gibberellin biosynthesis and the regulation of plant development.Plant Biol (Stuttg). 2006 May;8(3):281-90. doi: 10.1055/s-2006-923882. Plant Biol (Stuttg). 2006. PMID: 16807819 Review.
-
Regulation of gibberellin biosynthesis by light.Curr Opin Plant Biol. 1999 Oct;2(5):398-403. doi: 10.1016/s1369-5266(99)00012-6. Curr Opin Plant Biol. 1999. PMID: 10508758 Review.
Cited by
-
Identification of genes involved in metabolism and signalling of abscisic acid and gibberellins during Epimedium pseudowushanense B.L.Guo seed morphophysiological dormancy.Plant Cell Rep. 2018 Jul;37(7):1061-1075. doi: 10.1007/s00299-018-2291-8. Epub 2018 May 23. Plant Cell Rep. 2018. PMID: 29796945
-
Ectopic expression of pumpkin gibberellin oxidases alters gibberellin biosynthesis and development of transgenic Arabidopsis plants.Plant Physiol. 2006 Feb;140(2):528-36. doi: 10.1104/pp.105.073668. Epub 2005 Dec 29. Plant Physiol. 2006. PMID: 16384902 Free PMC article.
-
Genome-Wide Identification and Expression Analysis of ent-kaurene synthase-like Gene Family Associated with Abiotic Stress in Rice.Int J Mol Sci. 2024 May 18;25(10):5513. doi: 10.3390/ijms25105513. Int J Mol Sci. 2024. PMID: 38791550 Free PMC article.
-
The Current Status of Research on Gibberellin Biosynthesis.Plant Cell Physiol. 2020 Dec 23;61(11):1832-1849. doi: 10.1093/pcp/pcaa092. Plant Cell Physiol. 2020. PMID: 32652020 Free PMC article. Review.
-
Stamen-derived bioactive gibberellin is essential for male flower development of Cucurbita maxima L.J Exp Bot. 2012 Apr;63(7):2681-91. doi: 10.1093/jxb/err448. Epub 2012 Jan 20. J Exp Bot. 2012. PMID: 22268154 Free PMC article.
References
-
- Barlow P. The meristem and quiescent centre in cultured root apices of the gib-1 mutant of tomato (Lycopersicon esculentum Mill) Ann Bot. 1992;69:533–543.
-
- Blechschmidt S, Castel U, Gaskin P, Hedden P, Graebe JE, MacMillan J. GC/MS analysis of the plant hormones in seeds of Cucurbita maxima. Phytochemistry. 1984;23:553–558.
-
- Gaskin P, MacMillan J. GC-MS of Gibberellins and Related Compounds. Bristol, UK: Cantock's Enterprise; 1992.
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources

