A novel RING finger protein, human enhancer of invasion 10, alters mitotic progression through regulation of cyclin B levels
- PMID: 12612082
- PMCID: PMC149478
- DOI: 10.1128/MCB.23.6.2109-2122.2003
A novel RING finger protein, human enhancer of invasion 10, alters mitotic progression through regulation of cyclin B levels
Abstract
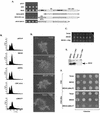
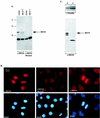
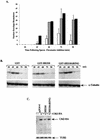
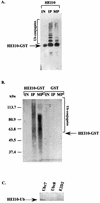
The process of cellular morphogenesis is highly conserved in eukaryotes and is dependent upon the function of proteins that are centrally involved in specification of the cell cycle. The human enhancer of invasion clone 10 (HEI10) protein was identified from a HeLa cell library based on its ability to promote yeast agar invasion and filamentation. Through two-hybrid screening, the mitotic cyclin B1 and an E2 ubiquitin-conjugating enzyme were isolated as HEI10-interacting proteins. Mutation of the HEI10 divergent RING finger motif (characteristic of E3 ubiquitin ligases) and Cdc2/cyclin binding and phosphorylation sites alter HEI10-dependent yeast phenotypes, including delay in G(2)/M transition. In vertebrates, the addition of HEI10 inhibits nuclear envelope breakdown and mitotic entry in Xenopus egg extracts. Mechanistically, HEI10 expression reduces cyclin B levels in cycling Xenopus eggs and reduces levels of the cyclin B ortholog Clb2p in yeast. HEI10 is itself a specific in vitro substrate of purified cyclin B/cdc2, with a TPVR motif as primary phosphorylation site. Finally, HEI10 is itself ubiquitinated in egg extracts and is also autoubiquitinated in vitro. These and other points lead to a model in which HEI10 defines a divergent class of E3 ubiquitin ligase, functioning in progression through G(2)/M.
Figures


Similar articles
-
Multisite phosphorylation of nuclear interaction partner of ALK (NIPA) at G2/M involves cyclin B1/Cdk1.J Biol Chem. 2007 Jun 1;282(22):15965-72. doi: 10.1074/jbc.M610819200. Epub 2007 Mar 27. J Biol Chem. 2007. PMID: 17389604
-
Two ubiquitin-conjugating enzymes, UbcP1/Ubc4 and UbcP4/Ubc11, have distinct functions for ubiquitination of mitotic cyclin.Mol Cell Biol. 2003 May;23(10):3497-505. doi: 10.1128/MCB.23.10.3497-3505.2003. Mol Cell Biol. 2003. PMID: 12724408 Free PMC article.
-
HEI10 negatively regulates cell invasion by inhibiting cyclin B/Cdk1 and other promotility proteins.Oncogene. 2007 Jul 19;26(33):4825-32. doi: 10.1038/sj.onc.1210282. Epub 2007 Feb 12. Oncogene. 2007. PMID: 17297447 Free PMC article.
-
SAG/ROC/Rbx/Hrt, a zinc RING finger gene family: molecular cloning, biochemical properties, and biological functions.Antioxid Redox Signal. 2001 Aug;3(4):635-50. doi: 10.1089/15230860152542989. Antioxid Redox Signal. 2001. PMID: 11554450 Review.
-
Mitotic entry: a matter of oscillating destruction.Cell Cycle. 2005 Nov;4(11):1515-7. doi: 10.4161/cc.4.11.2192. Epub 2005 Nov 21. Cell Cycle. 2005. PMID: 16258267 Review.
Cited by
-
HEI10 is subject to phase separation and mediates RPA1a degradation during meiotic interference-sensitive crossover formation.Proc Natl Acad Sci U S A. 2023 Dec 26;120(52):e2310542120. doi: 10.1073/pnas.2310542120. Epub 2023 Dec 22. Proc Natl Acad Sci U S A. 2023. PMID: 38134200 Free PMC article.
-
Visfatin Affects the Transcriptome of Porcine Luteal Cells during Early Pregnancy.Int J Mol Sci. 2024 Feb 16;25(4):2339. doi: 10.3390/ijms25042339. Int J Mol Sci. 2024. PMID: 38397019 Free PMC article.
-
Diverse roles for CDK-associated activity during spermatogenesis.FEBS Lett. 2019 Oct;593(20):2925-2949. doi: 10.1002/1873-3468.13627. Epub 2019 Oct 20. FEBS Lett. 2019. PMID: 31566717 Free PMC article. Review.
-
Mechanism and Disease Association With a Ubiquitin Conjugating E2 Enzyme: UBE2L3.Front Immunol. 2022 Feb 21;13:793610. doi: 10.3389/fimmu.2022.793610. eCollection 2022. Front Immunol. 2022. PMID: 35265070 Free PMC article. Review.
-
HEIP1 regulates crossover formation during meiosis in rice.Proc Natl Acad Sci U S A. 2018 Oct 16;115(42):10810-10815. doi: 10.1073/pnas.1807871115. Epub 2018 Oct 1. Proc Natl Acad Sci U S A. 2018. PMID: 30275327 Free PMC article.
References
-
- Ardley, H. C., N. G. Tan, S. A. Rose, A. F. Markham, and P. A. Robinson. 2001. Features of the parkin/ariadne-like ubiquitin ligase, HHARI, that regulate its interaction with the ubiquitin-conjugating enzyme, Ubch7. J. Biol. Chem. 276:19640-19647. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
