Chimeric and pseudotyped parvoviruses minimize the contamination of recombinant stocks with replication-competent viruses and identify a DNA sequence that restricts parvovirus H-1 in mouse cells
- PMID: 12610161
- PMCID: PMC149498
- DOI: 10.1128/jvi.77.6.3851-3858.2003
Chimeric and pseudotyped parvoviruses minimize the contamination of recombinant stocks with replication-competent viruses and identify a DNA sequence that restricts parvovirus H-1 in mouse cells
Abstract
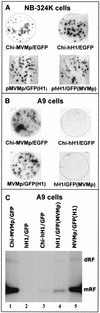
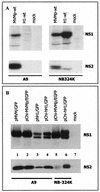
Recent studies demonstrated the ability of the recombinant autonomous parvoviruses MVMp (fibrotropic variant of the minute virus of mice) and H-1 to transduce therapeutic genes in tumor cells. However, recombinant vector stocks are contaminated by replication-competent viruses (RCVs) generated during the production procedure. To reduce the levels of RCVs, chimeric recombinant vector genomes were designed by replacing the right-hand region of H-1 virus DNA with that of the closely related MVMp virus DNA and conversely. Recombinant H-1 and MVMp virus pseudotypes were also produced with this aim. In both cases, the levels of RCVs contaminating the virus stocks were considerably reduced (virus was not detected in pseudotyped virus stocks, even after two amplification steps), while the yields of vector viruses produced were not affected. H-1 virus could be distinguished from MVMp virus by its restriction in mouse cells at an early stage of infection prior to detectable viral DNA replication and gene expression. The analysis of the composite viruses showed that this restriction could be assigned to a specific genomic determinant(s). Unlike MVMp virus, H-1 virus capsids were found to be a major determinant of the greater permissiveness of various human cell lines for this virus.
Figures

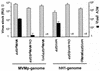
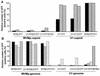
 , U373
, U373  , and HeLa
, and HeLa  ) after infection with the indicated recombinant virus stocks. (B) Relative numbers of GFP-positive mouse cells (A9
) after infection with the indicated recombinant virus stocks. (B) Relative numbers of GFP-positive mouse cells (A9  , L929
, L929  , Ehrlich ascitic fluid
, Ehrlich ascitic fluid  , and C3H10T1/2
, and C3H10T1/2  ) after infection with the indicated recombinant virus stocks.
) after infection with the indicated recombinant virus stocks.Similar articles
-
A novel MVMp-based vector system specifically designed to reduce the risk of replication-competent virus generation by homologous recombination.Gene Ther. 2001 Jun;8(12):921-9. doi: 10.1038/sj.gt.3301477. Gene Ther. 2001. PMID: 11426332
-
The infectivity and lytic activity of minute virus of mice wild-type and derived vector particles are strikingly different.J Virol. 2005 Jan;79(1):289-98. doi: 10.1128/JVI.79.1.289-298.2005. J Virol. 2005. PMID: 15596824 Free PMC article.
-
Construction and production of oncotropic vectors, derived from MVM(p), that share reduced sequence homology with helper plasmids.Cancer Gene Ther. 2002 Sep;9(9):762-70. doi: 10.1038/sj.cgt.7700496. Cancer Gene Ther. 2002. PMID: 12189526
-
Autonomous parvovirus vectors: preventing the generation of wild-type or replication-competent virus.J Gene Med. 2004 Feb;6 Suppl 1:S203-11. doi: 10.1002/jgm.504. J Gene Med. 2004. PMID: 14978763 Review.
-
Cancer gene therapy through autonomous parvovirus--mediated gene transfer.Curr Gene Ther. 2004 Sep;4(3):249-61. doi: 10.2174/1566523043346228. Curr Gene Ther. 2004. PMID: 15384939 Review.
Cited by
-
A nonproliferating parvovirus vaccine vector elicits sustained, protective humoral immunity following a single intravenous or intranasal inoculation.J Virol. 2004 Feb;78(3):1101-8. doi: 10.1128/jvi.78.3.1101-1108.2004. J Virol. 2004. PMID: 14722265 Free PMC article.
-
Mutations in the Non-Structural Protein-Coding Sequence of Protoparvovirus H-1PV Enhance the Fitness of the Virus and Show Key Benefits Regarding the Transduction Efficiency of Derived Vectors.Viruses. 2018 Mar 27;10(4):150. doi: 10.3390/v10040150. Viruses. 2018. PMID: 29584637 Free PMC article.
-
Pathology, organ distribution, and immune response after single and repeated intravenous injection of rats with clinical-grade parvovirus H1.Comp Med. 2015 Feb;65(1):23-35. Comp Med. 2015. PMID: 25730754 Free PMC article.
-
Oncolytic effects of parvovirus H-1 in medulloblastoma are associated with repression of master regulators of early neurogenesis.Int J Cancer. 2014 Feb 1;134(3):703-16. doi: 10.1002/ijc.28386. Epub 2013 Sep 2. Int J Cancer. 2014. PMID: 23852775 Free PMC article.
-
Through its nonstructural protein NS1, parvovirus H-1 induces apoptosis via accumulation of reactive oxygen species.J Virol. 2010 Jun;84(12):5909-22. doi: 10.1128/JVI.01797-09. Epub 2010 Apr 7. J Virol. 2010. PMID: 20375165 Free PMC article.
References
-
- Brown, C. S., F. M. DiSumma, J. Rommelaere, A. Y. Dege, J. J. Cornelis, C. Dinsart, and W. J. M. Spaan. 2002. Production of recombinant H1 parvovirus stocks devoid of replication-competent viruses. Hum. Gene Ther. 13:2135-2145. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

