Vaccinia virus G7L protein Interacts with the A30L protein and is required for association of viral membranes with dense viroplasm to form immature virions
- PMID: 12610117
- PMCID: PMC149536
- DOI: 10.1128/jvi.77.6.3418-3429.2003
Vaccinia virus G7L protein Interacts with the A30L protein and is required for association of viral membranes with dense viroplasm to form immature virions
Abstract
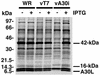
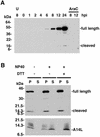
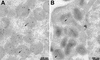
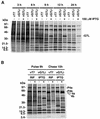
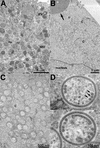
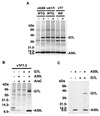
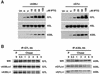
The vaccinia virus A30L protein is required for the association of electron-dense, granular, proteinaceous material with the concave surfaces of crescent membranes, an early step in viral morphogenesis. For the identification of additional proteins involved in this process, we used an antibody to the A30L protein, or to an epitope appended to its C terminus, to capture complexes from infected cells. A prominent 42-kDa protein was resolved and identified by mass spectrometry as the vaccinia virus G7L protein. This previously uncharacterized protein was expressed late in infection and was associated with immature virions and the cores of mature particles. In order to study the role of the G7L protein, a conditional lethal mutant was made by replacing the G7L gene with an inducible copy. Expression of G7L and formation of infectious virus was dependent on the addition of inducer. Under nonpermissive conditions, morphogenesis was blocked and viral crescent membranes and immature virions containing tubular elements were separated from the electron-dense granular viroplasm, which accumulated in large spherical masses. This phenotype was identical to that previously obtained with an inducible, conditional lethal A30L mutant. Additional in vivo and in vitro experiments provided evidence for the direct interaction of the A30L and G7L proteins and demonstrated that the stability of each one was dependent on its association with the other.
Figures



Similar articles
-
Vaccinia virus A30L protein is required for association of viral membranes with dense viroplasm to form immature virions.J Virol. 2001 Jul;75(13):5752-61. doi: 10.1128/JVI.75.13.5752-5761.2001. J Virol. 2001. PMID: 11390577 Free PMC article.
-
Physical and functional interactions between vaccinia virus F10 protein kinase and virion assembly proteins A30 and G7.J Virol. 2004 Jan;78(1):266-74. doi: 10.1128/jvi.78.1.266-274.2004. J Virol. 2004. PMID: 14671108 Free PMC article.
-
Vaccinia Virus A6 Is a Two-Domain Protein Requiring a Cognate N-Terminal Domain for Full Viral Membrane Assembly Activity.J Virol. 2017 Apr 28;91(10):e02405-16. doi: 10.1128/JVI.02405-16. Print 2017 May 15. J Virol. 2017. PMID: 28275183 Free PMC article.
-
Vaccinia virus proteolysis--a review.Rev Med Virol. 2006 May-Jun;16(3):187-202. doi: 10.1002/rmv.499. Rev Med Virol. 2006. PMID: 16710840 Free PMC article. Review.
-
Virus morphogenesis in the cell: methods and observations.Subcell Biochem. 2013;68:417-40. doi: 10.1007/978-94-007-6552-8_14. Subcell Biochem. 2013. PMID: 23737060 Free PMC article. Review.
Cited by
-
Cidofovir inhibits genome encapsidation and affects morphogenesis during the replication of vaccinia virus.J Virol. 2009 Nov;83(22):11477-90. doi: 10.1128/JVI.01061-09. Epub 2009 Sep 2. J Virol. 2009. PMID: 19726515 Free PMC article.
-
Bluetongue virus RNA binding protein NS2 is a modulator of viral replication and assembly.BMC Mol Biol. 2007 Jan 22;8:4. doi: 10.1186/1471-2199-8-4. BMC Mol Biol. 2007. PMID: 17241458 Free PMC article.
-
Vaccinia virus mutations in the L4R gene encoding a virion structural protein produce abnormal mature particles lacking a nucleocapsid.J Virol. 2014 Dec;88(24):14017-29. doi: 10.1128/JVI.02126-14. Epub 2014 Sep 24. J Virol. 2014. PMID: 25253347 Free PMC article.
-
Characterization of a vaccinia virus mutant with a deletion of the D10R gene encoding a putative negative regulator of gene expression.J Virol. 2006 Jan;80(2):553-61. doi: 10.1128/JVI.80.2.553-561.2006. J Virol. 2006. PMID: 16378957 Free PMC article.
-
Cell biological and functional characterization of the vaccinia virus F10 kinase: implications for the mechanism of virion morphogenesis.J Virol. 2005 Feb;79(4):2171-90. doi: 10.1128/JVI.79.4.2171-2190.2005. J Virol. 2005. PMID: 15681420 Free PMC article.
References
-
- Appleyard, G., A. J. Hapel, and E. A. Boulter. 1971. An antigenic difference between intracellular and extracellular rabbitpox virus. J. Gen. Virol. 13:9-17. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

