Unr is required in vivo for efficient initiation of translation from the internal ribosome entry sites of both rhinovirus and poliovirus
- PMID: 12610110
- PMCID: PMC149491
- DOI: 10.1128/jvi.77.6.3353-3359.2003
Unr is required in vivo for efficient initiation of translation from the internal ribosome entry sites of both rhinovirus and poliovirus
Abstract
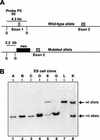
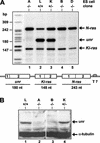
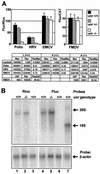
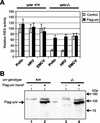
Translation of picornavirus RNAs is mediated by internal ribosomal entry site (IRES) elements and requires both standard eukaryotic translation initiation factors (eIFs) and IRES-specific cellular trans-acting factors (ITAFs). Unr, a cytoplasmic RNA-binding protein that contains five cold-shock domains and is encoded by the gene upstream of N-ras, stimulates translation directed by the human rhinovirus (HRV) IRES in vitro. To examine the role of Unr in translation of picornavirus RNAs in vivo, we derived murine embryonic stem (ES) cells in which either one (-/+) or both (-/-) copies of the unr gene were disrupted by homologous recombination. The activity of picornaviral IRES elements was analyzed in unr(+/+), unr(+/-), and unr(-/-) cell lines. Translation directed by the HRV IRES was severely impaired in unr(-/-) cells, as was that directed by the poliovirus IRES, revealing a requirement for Unr not previously observed in vitro. Transient expression of Unr in unr(-/-) cells efficiently restored the HRV and poliovirus IRES activities. In contrast, the IRES elements of encephalomyocarditis virus and foot-and-mouth-disease virus are not Unr dependent. Thus, Unr is a specific regulator of HRV and poliovirus translation in vivo and may represent a cell-specific determinant limiting replication of these viruses.
Figures
Similar articles
-
unr, a cellular cytoplasmic RNA-binding protein with five cold-shock domains, is required for internal initiation of translation of human rhinovirus RNA.Genes Dev. 1999 Feb 15;13(4):437-48. doi: 10.1101/gad.13.4.437. Genes Dev. 1999. PMID: 10049359 Free PMC article.
-
All five cold-shock domains of unr (upstream of N-ras) are required for stimulation of human rhinovirus RNA translation.J Gen Virol. 2004 Aug;85(Pt 8):2279-2287. doi: 10.1099/vir.0.80045-0. J Gen Virol. 2004. PMID: 15269369
-
Internal initiation of translation from the human rhinovirus-2 internal ribosome entry site requires the binding of Unr to two distinct sites on the 5' untranslated region.J Gen Virol. 2007 Nov;88(Pt 11):3043-3052. doi: 10.1099/vir.0.82463-0. J Gen Virol. 2007. PMID: 17947529
-
Elusive Trans-Acting Factors Which Operate with Type I (Poliovirus-like) IRES Elements.Int J Mol Sci. 2022 Dec 7;23(24):15497. doi: 10.3390/ijms232415497. Int J Mol Sci. 2022. PMID: 36555135 Free PMC article. Review.
-
Advances and Breakthroughs in IRES-Directed Translation and Replication of Picornaviruses.mBio. 2023 Apr 25;14(2):e0035823. doi: 10.1128/mbio.00358-23. Epub 2023 Mar 20. mBio. 2023. PMID: 36939331 Free PMC article. Review.
Cited by
-
Oncolytic virotherapy induced CSDE1 neo-antigenesis restricts VSV replication but can be targeted by immunotherapy.Nat Commun. 2021 Mar 26;12(1):1930. doi: 10.1038/s41467-021-22115-1. Nat Commun. 2021. PMID: 33772027 Free PMC article.
-
RNA Binding Proteins and Regulation of mRNA Translation in Erythropoiesis.Front Physiol. 2018 Jul 24;9:910. doi: 10.3389/fphys.2018.00910. eCollection 2018. Front Physiol. 2018. PMID: 30087616 Free PMC article. Review.
-
A peptide from autoantigen La blocks poliovirus and hepatitis C virus cap-independent translation and reveals a single tyrosine critical for La RNA binding and translation stimulation.J Virol. 2004 Apr;78(7):3763-76. doi: 10.1128/jvi.78.7.3763-3776.2004. J Virol. 2004. PMID: 15016896 Free PMC article.
-
Upstream of N-Ras C-terminal cold shock domains mediate poly(A) specificity in a novel RNA recognition mode and bind poly(A) binding protein.Nucleic Acids Res. 2023 Feb 28;51(4):1895-1913. doi: 10.1093/nar/gkac1277. Nucleic Acids Res. 2023. PMID: 36688322 Free PMC article.
-
SYNCRIP, a member of the heterogeneous nuclear ribonucleoprotein family, is involved in mouse hepatitis virus RNA synthesis.J Virol. 2004 Dec;78(23):13153-62. doi: 10.1128/JVI.78.23.13153-13162.2004. J Virol. 2004. PMID: 15542667 Free PMC article.
References
-
- Belsham, G. J., and R. J. Jackson. 2000. Translation initiation on picornavirus RNA. Translational control of gene expression. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
-
- Borman, A., M. T. Howell, J. G. Patton, and R. J. Jackson. 1993. The involvement of a spliceosome component in internal initiation of human rhinovirus RNA translation. J. Gen. Virol. 74:1775-1788. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

