Hematopoiesis-restricted minor histocompatibility antigens HA-1- or HA-2-specific T cells can induce complete remissions of relapsed leukemia
- PMID: 12601144
- PMCID: PMC151411
- DOI: 10.1073/pnas.0530192100
Hematopoiesis-restricted minor histocompatibility antigens HA-1- or HA-2-specific T cells can induce complete remissions of relapsed leukemia
Abstract
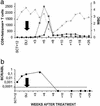
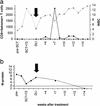
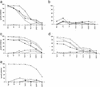
Donor lymphocyte infusion (DLI) into patients with a relapse of their leukemia or multiple myeloma after allogeneic stem cell transplantation (alloSCT) has been shown to be a successful treatment approach. The hematopoiesis-restricted minor histocompatibility antigens (mHAgs) HA-1 or HA-2 expressed on malignant cells of the recipient may serve as target antigens for alloreactive donor T cells. Recently we treated three mHAg HA-1- and/or HA-2-positive patients with a relapse of their disease after alloSCT with DLI from their mHAg HA-1- and/or HA-2-negative donors. Using HLA-A2HA-1 and HA-2 peptide tetrameric complexes we showed the emergence of HA-1- and HA-2-specific CD8(+) T cells in the blood of the recipients 5-7 weeks after DLI. The appearance of these tetramer-positive cells was followed immediately by a complete remission of the disease and restoration of 100% donor chimerism in each of the patients. Furthermore, cloned tetramer-positive T cells isolated during the clinical response specifically recognized HA-1 and HA-2 expressing malignant progenitor cells of the recipient and inhibited the growth of leukemic precursor cells in vitro. Thus, HA-1- and HA-2-specific cytotoxic T lymphocytes emerging in the blood of patients after DLI demonstrate graft-versus-leukemia or myeloma reactivity resulting in a durable remission. This finding implies that in vitro generated HA-1- and HA-2-specific cytotoxic T lymphocytes could be used as adoptive immunotherapy to treat hematological malignancies relapsing after alloSCT.
Figures


Similar articles
-
Direct cloning of leukemia-reactive T cells from patients treated with donor lymphocyte infusion shows a relative dominance of hematopoiesis-restricted minor histocompatibility antigen HA-1 and HA-2 specific T cells.Leukemia. 2004 Apr;18(4):798-808. doi: 10.1038/sj.leu.2403297. Leukemia. 2004. PMID: 14973499
-
A frameshift polymorphism in P2X5 elicits an allogeneic cytotoxic T lymphocyte response associated with remission of chronic myeloid leukemia.J Clin Invest. 2005 Dec;115(12):3506-16. doi: 10.1172/JCI24832. J Clin Invest. 2005. PMID: 16322791 Free PMC article.
-
Possible role of minor h antigens in the persistence of donor chimerism after stem cell transplantation; relevance for sustained leukemia remission.PLoS One. 2015 Mar 16;10(3):e0119595. doi: 10.1371/journal.pone.0119595. eCollection 2015. PLoS One. 2015. PMID: 25774796 Free PMC article.
-
[Minor antigens - major impact. The role of minor histocompatibility antigens in allogeneic hematopoietic stem cell transplantation].Dtsch Med Wochenschr. 2008 Jul;133(28-29):1511-6. doi: 10.1055/s-2008-1081100. Dtsch Med Wochenschr. 2008. PMID: 18597211 Review. German.
-
Minor histocompatibility antigens as targets of graft-versus-leukemia reactions.Curr Opin Hematol. 2002 Nov;9(6):497-502. doi: 10.1097/00062752-200211000-00005. Curr Opin Hematol. 2002. PMID: 12394171 Review.
Cited by
-
Developing strategies in the immunotherapy of leukemias.Cancer Control. 2013 Jan;20(1):49-59. doi: 10.1177/107327481302000108. Cancer Control. 2013. PMID: 23302907 Free PMC article. Review.
-
Cellular therapy following allogeneic stem-cell transplantation.Ther Adv Hematol. 2011 Dec;2(6):409-28. doi: 10.1177/2040620711412416. Ther Adv Hematol. 2011. PMID: 23556106 Free PMC article.
-
H60: A Unique Murine Hematopoietic Cell-Restricted Minor Histocompatibility Antigen for Graft-versus-Leukemia Effect.Front Immunol. 2020 Jun 10;11:1163. doi: 10.3389/fimmu.2020.01163. eCollection 2020. Front Immunol. 2020. PMID: 32587590 Free PMC article. Review.
-
The Graft-Versus-Leukemia Effect in AML.Front Oncol. 2019 Nov 19;9:1217. doi: 10.3389/fonc.2019.01217. eCollection 2019. Front Oncol. 2019. PMID: 31803612 Free PMC article. Review.
-
Naturally acquired tolerance and sensitization to minor histocompatibility antigens in healthy family members.Blood. 2009 Sep 10;114(11):2263-72. doi: 10.1182/blood-2009-01-200410. Epub 2009 Jun 8. Blood. 2009. PMID: 19506299 Free PMC article.
References
-
- Kolb H J, Mittermuller J, Clemm C, Holler E, Ledderose G, Brehm G, Heim M, Wilmanns W. Blood. 1990;76:2462–2465. - PubMed
-
- Kolb H J, Schattenberg A, Goldman J M, Hertenstein B, Jacobsen N, Arcese W, Ljungman P, Ferrant A, Verdonck L, Niederwieser D, et al. Blood. 1995;86:2041–2050. - PubMed
-
- Collins R H, Shpilberg O, Drobyski W R, Porter D L, Giralt S, Champlin R, Goodman S A, Wolff S N, Hu W, Verfaillie C, et al. J Clin Oncol. 1997;15:433–444. - PubMed
-
- Porter D L, Collins R H, Jr, Hardy C, Kernan N A, Drobyski W R, Giralt S, Flowers M E, Casper J, Leahey A, Parker P, et al. Blood. 2000;95:1214–1221. - PubMed
-
- Dazzi F, Szydlo R M, Craddock C, Cross N C, Kaeda J, Chase A, Olavarria E, van Rhee F, Kanfer E, Apperley J F, et al. Blood. 2000;95:67–71. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

