Unique appearance of proliferating antigen-presenting cells expressing DC-SIGN (CD209) in the decidua of early human pregnancy
- PMID: 12598322
- PMCID: PMC1868095
- DOI: 10.1016/S0002-9440(10)63884-9
Unique appearance of proliferating antigen-presenting cells expressing DC-SIGN (CD209) in the decidua of early human pregnancy
Abstract
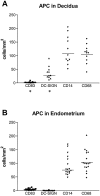
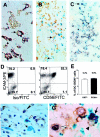
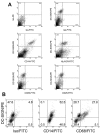
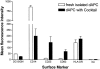
Intact human pregnancy can be regarded as an immunological paradox in that the maternal immune system accepts the allogeneic embryo without general immunosuppression. Because dendritic cell (DC) subsets could be involved in peripheral tolerance, the uterine mucosa (decidua) was investigated for DC populations. Here we describe the detailed immunohistochemical and functional characterization of HLA-DR-positive antigen-presenting cells (APCs) in early pregnancy decidua. In contrast to classical macrophages and CD83(+) DCs, which were found in comparable numbers in decidua and nonpregnant endometrium, only decidua harbored a significant population of HLA-DR(+)/DC-SIGN(+) APCs further phenotyped as CD14(+)/CD4(+)/CD68(+/-)/CD83(-)/CD25(-). These cells exhibited a remarkable proliferation rate (9.2 to 9.8% of all CD209(+) cells) by double staining with Ki67 and proliferating cell nuclear antigen. Unique within the DC-family, the majority of DC-SIGN(+) decidual APCs were observed in situ to have intimate contact with CD56(+)/CD16(-)/ICAM-3(+) decidual natural killer cells, another pregnancy-restricted cell population. In vitro, freshly isolated CD14(+)/DC-SIGN(+) decidual cells efficiently took up antigen, but could not stimulate naive allogeneic T cells at all. Treatment with an inflammatory cytokine cocktail resulted in down-regulation of antigen uptake capacity and evolving capacity to effectively stimulate resting T cells. Fluorescence-activated cell sorting analysis confirmed the maturation of CD14(+)/DC-SIGN(+) decidual cells into CD25(+)/CD83(+) mature DCs. In summary, this is the first identification of a uterine immature DC population expressing DC-SIGN, that appears only in pregnancy-associated tissue, has a high proliferation rate, and a conspicuous association with a natural killer subset.
Figures

Similar articles
-
Antigen-presenting cells in human endometrium during the menstrual cycle compared to early pregnancy.J Soc Gynecol Investig. 2004 Oct;11(7):488-93. doi: 10.1016/j.jsgi.2004.05.007. J Soc Gynecol Investig. 2004. PMID: 15458747
-
Altered decidual DC-SIGN+ antigen-presenting cells and impaired regulatory T-cell induction in preeclampsia.Am J Pathol. 2012 Dec;181(6):2149-60. doi: 10.1016/j.ajpath.2012.08.032. Epub 2012 Oct 9. Am J Pathol. 2012. PMID: 23063509
-
Apoptotic DC-SIGN+ cells in normal human decidua.Placenta. 2012 Apr;33(4):257-63. doi: 10.1016/j.placenta.2012.01.003. Epub 2012 Jan 18. Placenta. 2012. PMID: 22261157
-
Antigen presenting cells and HLA-G--a review.Placenta. 2005 Apr;26 Suppl A:S104-9. doi: 10.1016/j.placenta.2005.01.006. Placenta. 2005. PMID: 15837058 Review.
-
Antigen-presenting cells in the decidua.Chem Immunol Allergy. 2005;89:96-104. doi: 10.1159/000087951. Chem Immunol Allergy. 2005. PMID: 16129956 Review.
Cited by
-
Mucins help to avoid alloreactivity at the maternal fetal interface.Clin Dev Immunol. 2013;2013:542152. doi: 10.1155/2013/542152. Epub 2013 Jun 20. Clin Dev Immunol. 2013. PMID: 23864879 Free PMC article. Review.
-
Effects of heme oxygenase-1 on innate and adaptive immune responses promoting pregnancy success and allograft tolerance.Front Pharmacol. 2015 Jan 6;5:288. doi: 10.3389/fphar.2014.00288. eCollection 2014. Front Pharmacol. 2015. PMID: 25610397 Free PMC article. Review.
-
Perinatal Mesenchymal Stromal Cells and Their Possible Contribution to Fetal-Maternal Tolerance.Cells. 2019 Nov 7;8(11):1401. doi: 10.3390/cells8111401. Cells. 2019. PMID: 31703272 Free PMC article. Review.
-
Toll-like receptors at the maternal-fetal interface in normal pregnancy and pregnancy disorders.Am J Reprod Immunol. 2010 Jun;63(6):587-600. doi: 10.1111/j.1600-0897.2010.00848.x. Epub 2010 Mar 29. Am J Reprod Immunol. 2010. PMID: 20367625 Free PMC article. Review.
-
Maternal Obesity and the Uterine Immune Cell Landscape: The Shaping Role of Inflammation.Int J Mol Sci. 2020 May 27;21(11):3776. doi: 10.3390/ijms21113776. Int J Mol Sci. 2020. PMID: 32471078 Free PMC article. Review.
References
-
- Hart DN: Dendritic cells: unique leukocyte populations which control the primary immune response. Blood 1997, 90:3245-3287 - PubMed
-
- Steinman RM: The dendritic cell system and its role in immunogenicity. Annu Rev Immunol 1991, 9:271-296 - PubMed
-
- Thomas R, Davis LS, Lipsky PE: Comparative accessory cell function of human peripheral blood dendritic cells and monocytes. J Immunol 1993, 151:6840-6852 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

