War1p, a novel transcription factor controlling weak acid stress response in yeast
- PMID: 12588995
- PMCID: PMC151711
- DOI: 10.1128/MCB.23.5.1775-1785.2003
War1p, a novel transcription factor controlling weak acid stress response in yeast
Abstract
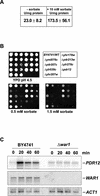
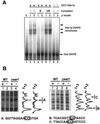
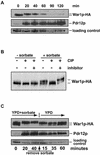
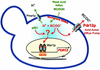
The Saccharomyces cerevisiae ATP-binding cassette (ABC) transporter Pdr12p effluxes weak acids such as sorbate and benzoate, thus mediating stress adaptation. In this study, we identify a novel transcription factor, War1p, as the regulator of this stress adaptation through transcriptional induction of PDR12. Cells lacking War1p are weak acid hypersensitive, since they fail to induce Pdr12p. The nuclear Zn2Cys6 transcriptional regulator War1p forms homodimers and is rapidly phosphorylated upon sorbate stress. The appearance of phosphorylated War1p isoforms coincides with transcriptional activation of PDR12. Promoter deletion analysis identified a novel cis-acting weak acid response element (WARE) in the PDR12 promoter required for PDR12 induction. War1p recognizes and decorates the WARE both in vitro and in vivo, as demonstrated by band shift assays and in vivo footprinting. Importantly, War1p occupies the WARE in the presence and absence of stress, demonstrating constitutive DNA binding in vivo. Our results suggest that weak acid stress triggers phosphorylation and perhaps activation of War1p. In turn, War1p activation is necessary for the induction of PDR12 through a novel signal transduction event that elicits weak organic acid stress adaptation.
Figures



Similar articles
-
A genetic screen identifies mutations in the yeast WAR1 gene, linking transcription factor phosphorylation to weak-acid stress adaptation.FEBS J. 2007 Jun;274(12):3094-107. doi: 10.1111/j.1742-4658.2007.05837.x. Epub 2007 May 17. FEBS J. 2007. PMID: 17509074
-
Global phenotypic analysis and transcriptional profiling defines the weak acid stress response regulon in Saccharomyces cerevisiae.Mol Biol Cell. 2004 Feb;15(2):706-20. doi: 10.1091/mbc.e03-05-0322. Epub 2003 Nov 14. Mol Biol Cell. 2004. PMID: 14617816 Free PMC article.
-
The pdr12 ABC transporter is required for the development of weak organic acid resistance in yeast.EMBO J. 1998 Aug 3;17(15):4257-65. doi: 10.1093/emboj/17.15.4257. EMBO J. 1998. PMID: 9687494 Free PMC article.
-
Carboxylic Acids Plasma Membrane Transporters in Saccharomyces cerevisiae.Adv Exp Med Biol. 2016;892:229-251. doi: 10.1007/978-3-319-25304-6_9. Adv Exp Med Biol. 2016. PMID: 26721276 Review.
-
Transcription factors and ABC transporters: from pleiotropic drug resistance to cellular signaling in yeast.FEBS Lett. 2020 Dec;594(23):3943-3964. doi: 10.1002/1873-3468.13964. Epub 2020 Nov 7. FEBS Lett. 2020. PMID: 33089887 Review.
Cited by
-
A yeast chemogenomic screen identifies pathways that modulate adipic acid toxicity.iScience. 2021 Mar 18;24(4):102327. doi: 10.1016/j.isci.2021.102327. eCollection 2021 Apr 23. iScience. 2021. PMID: 33889823 Free PMC article.
-
Black perithecial pigmentation in Fusarium species is due to the accumulation of 5-deoxybostrycoidin-based melanin.Sci Rep. 2016 May 19;6:26206. doi: 10.1038/srep26206. Sci Rep. 2016. PMID: 27193384 Free PMC article.
-
Positive-feedback, ratiometric biosensor expression improves high-throughput metabolite-producer screening efficiency in yeast.Synth Biol (Oxf). 2017 Jan 29;2(1):ysw002. doi: 10.1093/synbio/ysw002. eCollection 2017 Jan. Synth Biol (Oxf). 2017. PMID: 32995501 Free PMC article.
-
A mutation in Tac1p, a transcription factor regulating CDR1 and CDR2, is coupled with loss of heterozygosity at chromosome 5 to mediate antifungal resistance in Candida albicans.Genetics. 2006 Apr;172(4):2139-56. doi: 10.1534/genetics.105.054767. Epub 2006 Feb 1. Genetics. 2006. PMID: 16452151 Free PMC article.
-
Responses of Issatchenkia terricola WJL-G4 upon Citric Acid Stress.Molecules. 2022 Apr 21;27(9):2664. doi: 10.3390/molecules27092664. Molecules. 2022. PMID: 35566015 Free PMC article.
References
-
- Adams, A., D. E. Gottschling, C. A. Kaiser, and T. Stearns. 1997. Methods in yeast genetics: a laboratory course manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
-
- Balzi, E., M. Wang, S. Leterme, L. Van Dyck, and A. Goffeau. 1994. PDR5, a novel yeast multidrug resistance conferring transporter controlled by the transcription regulator PDR1. J. Biol. Chem. 269:2206-2214. - PubMed
-
- Bissinger, P. H., and K. Kuchler. 1994. Molecular cloning and expression of the Saccharomyces cerevisiae STS1 gene product. A yeast ABC transporter conferring mycotoxin resistance. J. Biol. Chem. 269:4180-4186. - PubMed
-
- Bracey, D., C. D. Holyoak, and P. J. Coote. 1998. Comparison of the inhibitory effect of sorbic acid and amphotericin B on Saccharomyces cerevisiae: is growth inhibition dependent on reduced intracellular pH? J. Appl. Microbiol. 85:1056-1066. - PubMed
-
- Cui, Z., T. Shiraki, D. Hirata, and T. Miyakawa. 1998. Yeast gene YRR1, which is required for resistance to 4-nitroquinoline N-oxide, mediates transcriptional activation of the multidrug resistance transporter gene SNQ2. Mol. Microbiol. 29:1307-1315. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
