The formation of skeletal muscle: from somite to limb
- PMID: 12587921
- PMCID: PMC1571050
- DOI: 10.1046/j.1469-7580.2003.00139.x
The formation of skeletal muscle: from somite to limb
Abstract
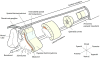
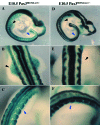
During embryogenesis, skeletal muscle forms in the vertebrate limb from progenitor cells originating in the somites. These cells delaminate from the hypaxial edge of the dorsal part of the somite, the dermomyotome, and migrate into the limb bud, where they proliferate, express myogenic determination factors and subsequently differentiate into skeletal muscle. A number of regulatory factors involved in these different steps have been identified. These include Pax3 with its target c-met, Lbx1 and Mox2 as well as the myogenic determination factors Myf5 and MyoD and factors required for differentiation such as Myogenin, Mrf4 and Mef2 isoforms. Mutants for genes such as Lbx1 and Mox2, expressed uniformly in limb muscle progenitors, reveal unexpected differences between fore and hind limb muscles, also indicated by the differential expression of Tbx genes. As development proceeds, a secondary wave of myogenesis takes place, and, postnatally, satellite cells become located under the basal lamina of adult muscle fibres. Satellite cells are thought to be the progenitor cells for adult muscle regeneration, during which similar genes to those which regulate myogenesis in the embryo also play a role. In particular, Pax3 as well as its orthologue Pax7 are important. The origin of secondary/fetal myoblasts and of adult satellite cells is unclear, as is the relation of the latter to so-called SP or stem cell populations, or indeed to potential mesangioblast progenitors, present in blood vessels. The oligoclonal origin of postnatal muscles points to a small number of founder cells, whether or not these have additional origins to the progenitor cells of the somite which form the first skeletal muscles, as discussed here for the embryonic limb.
Figures


Similar articles
-
[Early stages of myogenesis as seen through the action of the myf-5 gene].C R Seances Soc Biol Fil. 1997;191(1):43-54. C R Seances Soc Biol Fil. 1997. PMID: 9181127 French.
-
The homeobox gene Msx1 is expressed in a subset of somites, and in muscle progenitor cells migrating into the forelimb.Development. 1999 Jun;126(12):2689-701. doi: 10.1242/dev.126.12.2689. Development. 1999. PMID: 10331980
-
Eya1 and Eya2 proteins are required for hypaxial somitic myogenesis in the mouse embryo.Dev Biol. 2007 Feb 15;302(2):602-16. doi: 10.1016/j.ydbio.2006.08.059. Epub 2006 Sep 1. Dev Biol. 2007. PMID: 17098221
-
Myogenic regulatory factors and the specification of muscle progenitors in vertebrate embryos.Annu Rev Cell Dev Biol. 2002;18:747-83. doi: 10.1146/annurev.cellbio.18.012502.105758. Epub 2002 Apr 2. Annu Rev Cell Dev Biol. 2002. PMID: 12142270 Review.
-
Function of the myogenic regulatory factors Myf5, MyoD, Myogenin and MRF4 in skeletal muscle, satellite cells and regenerative myogenesis.Semin Cell Dev Biol. 2017 Dec;72:19-32. doi: 10.1016/j.semcdb.2017.11.011. Epub 2017 Nov 15. Semin Cell Dev Biol. 2017. PMID: 29127046 Review.
Cited by
-
Muscle cell-derived factors inhibit inflammatory stimuli-induced damage in hMSC-derived chondrocytes.Osteoarthritis Cartilage. 2013 Jul;21(7):990-8. doi: 10.1016/j.joca.2013.04.011. Epub 2013 Apr 20. Osteoarthritis Cartilage. 2013. PMID: 23611899 Free PMC article.
-
Satellite cells and the muscle stem cell niche.Physiol Rev. 2013 Jan;93(1):23-67. doi: 10.1152/physrev.00043.2011. Physiol Rev. 2013. PMID: 23303905 Free PMC article. Review.
-
Epigenetics of Genes Preferentially Expressed in Dissimilar Cell Populations: Myoblasts and Cerebellum.Epigenomes. 2024 Jan 26;8(1):4. doi: 10.3390/epigenomes8010004. Epigenomes. 2024. PMID: 38390894 Free PMC article.
-
Interplay of Nkx3.2, Sox9 and Pax3 regulates chondrogenic differentiation of muscle progenitor cells.PLoS One. 2012;7(7):e39642. doi: 10.1371/journal.pone.0039642. Epub 2012 Jul 2. PLoS One. 2012. PMID: 22768305 Free PMC article.
-
Intrinsic epigenetic regulation of the D4Z4 macrosatellite repeat in a transgenic mouse model for FSHD.PLoS Genet. 2013 Apr;9(4):e1003415. doi: 10.1371/journal.pgen.1003415. Epub 2013 Apr 4. PLoS Genet. 2013. PMID: 23593020 Free PMC article.
References
-
- Beauchamp JR, Heslop L, Yu DSW, Kelly RG, Tajbakhsh T, Buckingham ME, et al. Expression of CD34 and Myf5 defines the majority of quiescent adult skeletal muscle satellite cells. J. Cell Biol. 2000;151:1221–1233. 10.1046/j.1469-7580.2003.00139.x. - DOI - PMC - PubMed
-
- Black BL, Olson EN. Transcriptional control of muscle development by myocyte enhancer factor 2 (MEF2) proteins. Ann. Rev. Cell Dev. Biol. 1998;14:167–196. 10.1046/j.1469-7580.2003.00139.x. - DOI - PubMed
-
- Bladt F, Riethmacher D, Isenmann S, Aguzzi A, Birchmeier C. Essential role for the c-met receptor in the migration of myogenic precursor cells into the limb bud. Nature. 1995;376:768–771. 10.1046/j.1469-7580.2003.00139.x. - DOI - PubMed
-
- Buckingham M. Skeletal muscle formation in vertebrates. Current Opinion Genet. Dev. 2001;11:440–448. 10.1046/j.1469-7580.2003.00139.x. - DOI - PubMed
-
- Christ B, Ordahl CP. Early stages of chick somite development. Anat. Embryol. 1995;191:381–396. 10.1046/j.1469-7580.2003.00139.x. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

