Envelopment of human cytomegalovirus occurs by budding into Golgi-derived vacuole compartments positive for gB, Rab 3, trans-golgi network 46, and mannosidase II
- PMID: 12584343
- PMCID: PMC149787
- DOI: 10.1128/jvi.77.5.3191-3203.2003
Envelopment of human cytomegalovirus occurs by budding into Golgi-derived vacuole compartments positive for gB, Rab 3, trans-golgi network 46, and mannosidase II
Erratum in
- J Virol. Arch. 2003 Jul;77(14):8179
Abstract
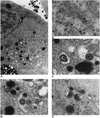
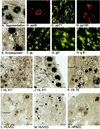
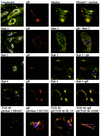
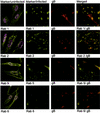
Although considerable progress has been made towards characterizing virus assembly processes, assignment of the site of tegumentation and envelopment for human cytomegalovirus (HCMV) is still not clear. In this study, we examined the envelopment of HCMV particles in human lung fibroblasts (HF) HL 411 and HL 19, human umbilical vein endothelial cells, human pulmonary arterial endothelial cells, and arterial smooth muscle cells at different time points after infection by electron microscopy (EM), immunohistochemistry, and confocal microscopy analysis. Double-immunofluorescence labeling experiments demonstrated colocalization of the HCMV glycoprotein B (gB) with the Golgi resident enzyme mannosidase II, the Golgi marker TGN (trans-Golgi network) 46, and the secretory vacuole marker Rab 3 in all cell types investigated. Final envelopment of tegumented capsids was observed at 5 days postinfection by EM, when tegumented capsids budded into subcellular compartments located in the cytoplasm, in close proximity to the Golgi apparatus. Immunogold labeling and EM analysis confirmed staining of the budding compartment with HCMV gB, Rab 3, and mannosidase II in HL 411 cells. However, the markers Rab 1, Rab 2, Rab 7, Lamp 1 (late endosomes and lysosomes), and Lamp 2 (lysosomes) neither showed specific staining of the budding compartment in the immunogold labeling experiments nor colocalized with gB in the immunofluorescent colocalization experiments in any cell type studied. Together, these results suggest that the final envelopment of HCMV particles takes place mainly into a Golgi-derived secretory vacuole destined for the plasma membrane, which may release new infectious virus particles by fusion with the plasma membrane.
Figures
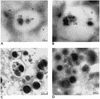
Similar articles
-
Accumulation of virion tegument and envelope proteins in a stable cytoplasmic compartment during human cytomegalovirus replication: characterization of a potential site of virus assembly.J Virol. 2000 Jan;74(2):975-86. doi: 10.1128/jvi.74.2.975-986.2000. J Virol. 2000. PMID: 10623760 Free PMC article.
-
Phosphorylation of human cytomegalovirus glycoprotein B (gB) at the acidic cluster casein kinase 2 site (Ser900) is required for localization of gB to the trans-Golgi network and efficient virus replication.J Virol. 2004 Jan;78(1):285-93. doi: 10.1128/jvi.78.1.285-293.2004. J Virol. 2004. PMID: 14671110 Free PMC article.
-
Spatial relationships between markers for secretory and endosomal machinery in human cytomegalovirus-infected cells versus those in uninfected cells.J Virol. 2011 Jun;85(12):5864-79. doi: 10.1128/JVI.00155-11. Epub 2011 Apr 6. J Virol. 2011. PMID: 21471245 Free PMC article.
-
Host Cell Signatures of the Envelopment Site within Beta-Herpes Virions.Int J Mol Sci. 2022 Sep 1;23(17):9994. doi: 10.3390/ijms23179994. Int J Mol Sci. 2022. PMID: 36077391 Free PMC article. Review.
-
Herpesvirus Nuclear Egress across the Outer Nuclear Membrane.Viruses. 2021 Nov 24;13(12):2356. doi: 10.3390/v13122356. Viruses. 2021. PMID: 34960625 Free PMC article. Review.
Cited by
-
CD13/aminopeptidase N and murine cytomegalovirus infection.Virology. 2005 Mar 30;334(1):1-9. doi: 10.1016/j.virol.2005.01.028. Virology. 2005. PMID: 15749117 Free PMC article.
-
The endoplasmic reticulum chaperone BiP/GRP78 is important in the structure and function of the human cytomegalovirus assembly compartment.J Virol. 2009 Nov;83(22):11421-8. doi: 10.1128/JVI.00762-09. Epub 2009 Sep 9. J Virol. 2009. PMID: 19741001 Free PMC article.
-
Role of the endoplasmic reticulum chaperone BiP, SUN domain proteins, and dynein in altering nuclear morphology during human cytomegalovirus infection.J Virol. 2010 Jul;84(14):7005-17. doi: 10.1128/JVI.00719-10. Epub 2010 May 19. J Virol. 2010. PMID: 20484513 Free PMC article.
-
The tegument protein UL71 of human cytomegalovirus is involved in late envelopment and affects multivesicular bodies.J Virol. 2011 Apr;85(8):3821-32. doi: 10.1128/JVI.01540-10. Epub 2011 Feb 2. J Virol. 2011. PMID: 21289123 Free PMC article.
-
Secondary Envelopment of Human Cytomegalovirus Is a Fast Process Utilizing the Endocytic Compartment as a Major Membrane Source.Biomolecules. 2024 Sep 12;14(9):1149. doi: 10.3390/biom14091149. Biomolecules. 2024. PMID: 39334915 Free PMC article.
References
-
- Avitabile, E., P. L. Ward, C. Di Lazzaro, M. R. Torrisi, B. Roizman, and G. Campadelli-Fiume. 1994. The herpes simplex virus UL20 protein compensates for the differential disruption of exocytosis of virions and viral membrane glycoproteins associated with fragmentation of the Golgi apparatus. J. Virol. 68:7397-7405. - PMC - PubMed
-
- Brideau, A. D., L. W. Enquist, and R. S. Tirabassi. 2000. The role of virion membrane protein endocytosis in the herpesvirus life cycle. J. Clin. Virol. 17:69-82. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

