Local routes revisited: the space and time dependence of the Ca2+ signal for phasic transmitter release at the rat calyx of Held
- PMID: 12562955
- PMCID: PMC2342725
- DOI: 10.1113/jphysiol.2002.032714
Local routes revisited: the space and time dependence of the Ca2+ signal for phasic transmitter release at the rat calyx of Held
Abstract
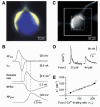
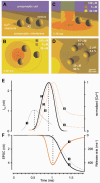
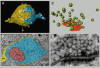
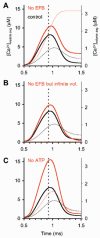
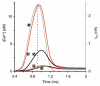
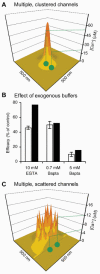
During the last decade, advances in experimental techniques and quantitative modelling have resulted in the development of the calyx of Held as one of the best preparations in which to study synaptic transmission. Here we review some of these advances, including simultaneous recording of pre- and postsynaptic currents, measuring the Ca2+ sensitivity of transmitter release, reconstructing the 3-D anatomy at the electron microscope (EM) level, and modelling the buffered diffusion of Ca2+ in the nerve terminal. An important outcome of these studies is an improved understanding of the Ca2+ signal that controls phasic transmitter release. This article illustrates the spatial and temporal aspects of the three main steps in the presynaptic signalling cascade: Ca2+ influx through voltage-gated calcium channels, buffered Ca2+ diffusion from the channels to releasable vesicles, and activation of the Ca2+ sensor for release. Particular emphasis is placed on how presynaptic Ca2+ buffers affect the Ca2+ signal and thus the amplitude and time course of the release probability. Since many aspects of the signalling cascade were first conceived with reference to the squid giant presynaptic terminal, we include comparisons with the squid model and revisit some of its implications. Whilst the characteristics of buffered Ca2+ diffusion presented here are based on the calyx of Held, we demonstrate the circumstances under which they may be valid for other nerve terminals at mammalian CNS synapses.
Figures

Similar articles
-
Calcium secretion coupling at calyx of Held governed by nonuniform channel-vesicle topography.J Neurosci. 2002 Mar 1;22(5):1648-67. doi: 10.1523/JNEUROSCI.22-05-01648.2002. J Neurosci. 2002. PMID: 11880495 Free PMC article.
-
The timing of phasic transmitter release is Ca2+-dependent and lacks a direct influence of presynaptic membrane potential.Proc Natl Acad Sci U S A. 2003 Dec 9;100(25):15200-5. doi: 10.1073/pnas.2433276100. Epub 2003 Nov 20. Proc Natl Acad Sci U S A. 2003. PMID: 14630950 Free PMC article.
-
Presynaptic Ca2+ requirements and developmental regulation of posttetanic potentiation at the calyx of Held.J Neurosci. 2005 May 25;25(21):5127-37. doi: 10.1523/JNEUROSCI.1295-05.2005. J Neurosci. 2005. PMID: 15917453 Free PMC article.
-
Presynaptic Ca2+ dynamics, Ca2+ buffers and synaptic efficacy.Cell Calcium. 2005 May;37(5):489-95. doi: 10.1016/j.ceca.2005.01.003. Cell Calcium. 2005. PMID: 15820398 Review.
-
Presynaptic Ca2+ channels--integration centers for neuronal signaling pathways.Trends Neurosci. 2006 Nov;29(11):617-24. doi: 10.1016/j.tins.2006.08.006. Epub 2006 Aug 30. Trends Neurosci. 2006. PMID: 16942804 Review.
Cited by
-
Calcium channels: unanswered questions.J Bioenerg Biomembr. 2003 Dec;35(6):461-75. doi: 10.1023/b:jobb.0000008020.86004.28. J Bioenerg Biomembr. 2003. PMID: 15000516 Review.
-
Synapsin-regulated synaptic transmission from readily releasable synaptic vesicles in excitatory hippocampal synapses in mice.J Physiol. 2006 Feb 15;571(Pt 1):75-82. doi: 10.1113/jphysiol.2005.100685. Epub 2005 Dec 1. J Physiol. 2006. PMID: 16322053 Free PMC article.
-
Control of neurotransmitter release: From Ca2+ to voltage dependent G-protein coupled receptors.Pflugers Arch. 2010 Nov;460(6):975-90. doi: 10.1007/s00424-010-0872-7. Epub 2010 Sep 2. Pflugers Arch. 2010. PMID: 20811904 Review.
-
Stochastic models for the in silico simulation of synaptic processes.BMC Bioinformatics. 2008 Apr 25;9 Suppl 4(Suppl 4):S7. doi: 10.1186/1471-2105-9-S4-S7. BMC Bioinformatics. 2008. PMID: 18460180 Free PMC article.
-
A dual-Ca2+-sensor model for neurotransmitter release in a central synapse.Nature. 2007 Nov 29;450(7170):676-82. doi: 10.1038/nature06308. Nature. 2007. PMID: 18046404 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

