Molecular genetic analysis of a group A Streptococcus operon encoding serum opacity factor and a novel fibronectin-binding protein, SfbX
- PMID: 12562790
- PMCID: PMC142848
- DOI: 10.1128/JB.185.4.1208-1217.2003
Molecular genetic analysis of a group A Streptococcus operon encoding serum opacity factor and a novel fibronectin-binding protein, SfbX
Abstract
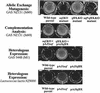
The group A Streptococcus (GAS) sof gene encodes the serum opacity factor protein, which is capable of opacifying mammalian sera and binding at least two host proteins, fibronectin and fibrinogen. The sof gene exists in approximately 50% of clinical isolates, and there is a classical association of so-called nephritogenic strains with the opacity factor-positive phenotype. In both a type emm49 strain and a type emm12 strain, the sequences upstream of the 5' end of sof and downstream of the putative terminator were determined to be nearly identical to a region in the M type 1 genome approximately 10 kb upstream of the emm1 gene. This close genetic linkage is likely reflected in the strict correlation of opacity factor phenotype with specific emm genotypes. A new fibronectin-binding protein gene, sfbX, was discovered immediately downstream of sof in emm12 and emm49 strains and in several other sof-positive strains. The sof and sfbX genes were found to be expressed on the same transcription unit, which was correlated with the putative promoter and rho-independant terminator sequences that flank these two genes. The sfbX genes from different emm types are predicted to encode approximately 650-residue surface-bound proteins sharing 89 to 92% sequence identity. SfbX residues approximately 1 to 480 are not highly similar to those of other known proteins, with the closest match being the Staphylococcus aureus coagulase protein. The remaining portions of these proteins (residues 481 to 650) contain four putative fibronectin-binding repeats highly similar to those of other streptococcal fibronectin-binding proteins and a potential LP(X)SG cell wall anchor motif. Targeted in-frame allelic-exchange mutagenesis, complementation, and heterologous-expression studies found that serum opacification is encoded by sof alone and that sfbX encodes a fibronectin-binding function. A recombinant SfbX protein was found to bind immobilized fibronectin and to partially inhibit GAS adherence to fibronectin. The sfbX gene was found to be present only in sof-positive strains, and together these genes could influence the spectrum of tissues colonized by sof-positive GAS.
Figures



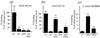
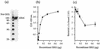
Similar articles
-
Serum opacity factor promotes group A streptococcal epithelial cell invasion and virulence.Mol Microbiol. 2006 Oct;62(1):15-25. doi: 10.1111/j.1365-2958.2006.05337.x. Epub 2006 Aug 30. Mol Microbiol. 2006. PMID: 16942605
-
emm and sof gene sequence variation in relation to serological typing of opacity-factor-positive group A streptococci.Microbiology (Reading). 2000 May;146 ( Pt 5):1195-1209. doi: 10.1099/00221287-146-5-1195. Microbiology (Reading). 2000. PMID: 10832648
-
Mga is sufficient to activate transcription in vitro of sof-sfbX and other Mga-regulated virulence genes in the group A Streptococcus.J Bacteriol. 2006 Mar;188(6):2038-47. doi: 10.1128/JB.188.6.2038-2047.2006. J Bacteriol. 2006. PMID: 16513733 Free PMC article.
-
The structure and function of serum opacity factor: a unique streptococcal virulence determinant that targets high-density lipoproteins.J Biomed Biotechnol. 2010;2010:956071. doi: 10.1155/2010/956071. Epub 2010 Jul 8. J Biomed Biotechnol. 2010. PMID: 20671930 Free PMC article. Review.
-
Molecular Basis of Serotyping and the Underlying Genetic Organization of Streptococcus pyogenes.2016 Feb 10 [updated 2016 Mar 25]. In: Ferretti JJ, Stevens DL, Fischetti VA, editors. Streptococcus pyogenes: Basic Biology to Clinical Manifestations [Internet]. Oklahoma City (OK): University of Oklahoma Health Sciences Center; 2016–. 2016 Feb 10 [updated 2016 Mar 25]. In: Ferretti JJ, Stevens DL, Fischetti VA, editors. Streptococcus pyogenes: Basic Biology to Clinical Manifestations [Internet]. Oklahoma City (OK): University of Oklahoma Health Sciences Center; 2016–. PMID: 26866230 Free Books & Documents. Review.
Cited by
-
The staphylocoagulase family of zymogen activator and adhesion proteins.Cell Mol Life Sci. 2004 Nov;61(22):2793-8. doi: 10.1007/s00018-004-4285-7. Cell Mol Life Sci. 2004. PMID: 15558209 Free PMC article.
-
The CiaR response regulator in group B Streptococcus promotes intracellular survival and resistance to innate immune defenses.J Bacteriol. 2009 Apr;191(7):2023-32. doi: 10.1128/JB.01216-08. Epub 2008 Dec 29. J Bacteriol. 2009. PMID: 19114476 Free PMC article.
-
Study of streptococcal hemoprotein receptor (Shr) in iron acquisition and virulence of M1T1 group A streptococcus.Virulence. 2012 Nov 15;3(7):566-75. doi: 10.4161/viru.21933. Epub 2012 Oct 17. Virulence. 2012. PMID: 23076332 Free PMC article.
-
Growth phase-dependent modulation of Rgg binding specificity in Streptococcus pyogenes.J Bacteriol. 2012 Aug;194(15):3961-71. doi: 10.1128/JB.06709-11. Epub 2012 May 25. J Bacteriol. 2012. PMID: 22636768 Free PMC article.
-
Use of flow cytometry for the adhesion analysis of Streptococcus pyogenes mutant strains to epithelial cells: investigation of the possible role of surface pullulanase and cysteine protease, and the transcriptional regulator Rgg.BMC Microbiol. 2006 Feb 24;6:18. doi: 10.1186/1471-2180-6-18. BMC Microbiol. 2006. PMID: 16504124 Free PMC article.
References
-
- Beall, B., G. Gherardi, M. Lovgren, R. R. Facklam, B. A. Forwick, and G. J. Tyrrell. 2000. emm and sof gene sequence variation in relation to serological typing of opacity-factor-positive group A streptococci. Microbiology 146:1195-1209. - PubMed
-
- Beres, S. B., G. L. Sylva, K. D. Barbian, B. Lei, J. S. Hoff, N. D. Mammarella, M. Y. Liu, J. C. Smoot, S. F. Porcella, L. D. Parkins, D. S. Campbell, T. M. Smith, J. K. McCormick, D. Y. Leung, P. M. Schlievert, and J. M. Musser. 2002. Genome sequence of a serotype M3 strain of group A Streptococcus: phage-encoded toxins, the high-virulence phenotype, and clone emergence. Proc. Natl. Acad. Sci. USA 99:10078-10083. - PMC - PubMed
-
- Bessen, D. E., C. M. Sotir, T. L. Readdy, and S. K. Hollingshead. 1996. Genetic correlates of throat and skin isolates of group A streptococci. J. Infect. Dis. 173:896-900. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources

