The Helicobacter pylori CagA protein induces cortactin dephosphorylation and actin rearrangement by c-Src inactivation
- PMID: 12554652
- PMCID: PMC140734
- DOI: 10.1093/emboj/cdg050
The Helicobacter pylori CagA protein induces cortactin dephosphorylation and actin rearrangement by c-Src inactivation
Abstract
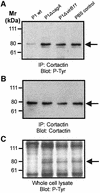
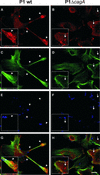
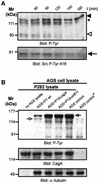
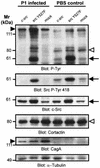
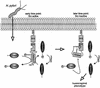
The gastric pathogen Helicobacter pylori translocates the CagA protein into epithelial cells by a type IV secretion process. Translocated CagA is tyrosine phosphorylated (CagA(P-Tyr)) on specific EPIYA sequence repeats by Src family tyrosine kinases. Phos phorylation of CagA induces the dephosphorylation of as yet unidentified cellular proteins, rearrangements of the host cell actin cytoskeleton and cell scattering. We show here that CagA(P-Tyr) inhibits the catalytic activity of c-Src in vivo and in vitro. c-Src inactivation leads to tyrosine dephosphorylation of the actin binding protein cortactin. Concomitantly, cortactin is specifically redistributed to actin-rich cellular protrusions. c-Src inactivation and cortactin dephosphorylation are required for rearrangements of the actin cytoskeleton. Moreover, CagA(P-Tyr)-mediated c-Src inhibition downregulates further CagA phosphorylation through a negative feedback loop. This is the first report of a bacterial virulence factor that inhibits signalling of a eukaryotic tyrosine kinase and on a role of c-Src inactivation in host cell cytoskeletal rearrangements.
Figures



Similar articles
-
Role of Abl and Src family kinases in actin-cytoskeletal rearrangements induced by the Helicobacter pylori CagA protein.Eur J Cell Biol. 2011 Nov;90(11):880-90. doi: 10.1016/j.ejcb.2010.11.006. Epub 2011 Jan 17. Eur J Cell Biol. 2011. PMID: 21247656 Review.
-
Activation of Abl by Helicobacter pylori: a novel kinase for CagA and crucial mediator of host cell scattering.Gastroenterology. 2007 Apr;132(4):1309-19. doi: 10.1053/j.gastro.2007.01.050. Epub 2007 Feb 1. Gastroenterology. 2007. PMID: 17408661
-
Interaction between the Helicobacter pylori CagA and alpha-Pix in gastric epithelial AGS cells.Ann N Y Acad Sci. 2007 Jan;1096:18-23. doi: 10.1196/annals.1397.065. Ann N Y Acad Sci. 2007. PMID: 17405911
-
Influence of EPIYA-repeat polymorphism on the phosphorylation-dependent biological activity of Helicobacter pylori CagA.Gastroenterology. 2006 Apr;130(4):1181-90. doi: 10.1053/j.gastro.2005.12.038. Gastroenterology. 2006. PMID: 16618412
-
The versatility of Helicobacter pylori CagA effector protein functions: The master key hypothesis.Helicobacter. 2010 Jun;15(3):163-76. doi: 10.1111/j.1523-5378.2010.00759.x. Helicobacter. 2010. PMID: 20557357 Review.
Cited by
-
H pylori and host interactions that influence pathogenesis.World J Gastroenterol. 2006 Sep 21;12(35):5599-605. doi: 10.3748/wjg.v12.i35.5599. World J Gastroenterol. 2006. PMID: 17007010 Free PMC article. Review.
-
Cortactin: A Major Cellular Target of the Gastric Carcinogen Helicobacter pylori.Cancers (Basel). 2020 Jan 9;12(1):159. doi: 10.3390/cancers12010159. Cancers (Basel). 2020. PMID: 31936446 Free PMC article. Review.
-
Exploring alternative treatments for Helicobacter pylori infection.World J Gastroenterol. 2014 Feb 14;20(6):1450-69. doi: 10.3748/wjg.v20.i6.1450. World J Gastroenterol. 2014. PMID: 24587621 Free PMC article. Review.
-
The beta1 integrin activates JNK independent of CagA, and JNK activation is required for Helicobacter pylori CagA+-induced motility of gastric cancer cells.J Biol Chem. 2008 May 16;283(20):13952-63. doi: 10.1074/jbc.M800289200. Epub 2008 Mar 20. J Biol Chem. 2008. PMID: 18356158 Free PMC article.
-
Repressed TGF-β signaling through CagA-Smad3 interaction as pathogenic mechanisms of Helicobacter pylori-associated gastritis.J Clin Biochem Nutr. 2015 Sep;57(2):113-20. doi: 10.3164/jcbn.15-38. Epub 2015 Jul 30. J Clin Biochem Nutr. 2015. PMID: 26388668 Free PMC article.
References
-
- Akopyants N.S. et al. (1998) Analyses of the cag pathogenicity island of Helicobacter pylori. Mol. Microbiol., 28, 37–53. - PubMed
-
- Backert S., Ziska,E., Brinkmann,V., Zimny-Arndt,U., Fauconnier,A., Jungblut,P.R., Naumann,M. and Meyer,T.F. (2000) Translocation of the Helicobacter pylori CagA protein in gastric epithelial cells by a type IV secretion apparatus. Cell Microbiol., 2, 155–164. - PubMed
-
- Backert S., Moese,S., Selbach,M., Brinkmann,V. and Meyer,T.F. (2001) Phosphorylation of tyrosine 972 of the Helicobacter pylori CagA protein is essential for induction of a scattering phenotype in gastric epithelial cells. Mol. Microbiol., 42, 631–644. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

