G protein-dependent CCR5 signaling is not required for efficient infection of primary T lymphocytes and macrophages by R5 human immunodeficiency virus type 1 isolates
- PMID: 12551993
- PMCID: PMC141084
- DOI: 10.1128/jvi.77.4.2550-2558.2003
G protein-dependent CCR5 signaling is not required for efficient infection of primary T lymphocytes and macrophages by R5 human immunodeficiency virus type 1 isolates
Abstract
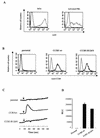
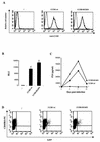
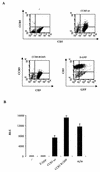
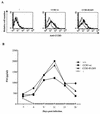
The requirement of human immunodeficiency virus (HIV)-induced CCR5 activation for infection by R5 HIV type 1 (HIV-1) strains remains controversial. Ectopic CCR5 expression in CD4(+)-transformed cells or pharmacological inhibition of G(alpha)i proteins coupled to CCR5 left unsolved whether CCR5-dependent cell activation is necessary for the HIV life cycle. In this study, we investigated the role played by HIV-induced CCR5-dependent cell signaling during infection of primary CD4-expressing leukocytes. Using lentiviral vectors, we restored CCR5 expression in T lymphocytes and macrophages from individuals carrying the homozygous 32-bp deletion of the CCR5 gene (ccr5 Delta32/Delta32). Expression of wild-type (wt) CCR5 in ccr5 Delta32/Delta32 cells permitted infection by R5 HIV isolates. We assessed the capacity of a CCR5 derivative carrying a mutated DRY motif (CCR5-R126N) in the second intracellular loop to work as an HIV-1 coreceptor. The R126N mutation is known to disable G protein coupling and agonist-induced signal transduction through CCR5 and other G protein-coupled receptors. Despite its inability to promote either intracellular calcium mobilization or cell chemotaxis, the inactive CCR5-R126N mutant provided full coreceptor function to several R5 HIV-1 isolates in primary cells as efficiently as wt CCR5. We conclude that in a primary, CCR5-reconstituted CD4(+) cell environment, G protein signaling is dispensable for R5 HIV-1 isolates to actively infect primary CD4(+) T lymphocytes or macrophages.
Figures
Similar articles
-
G-protein signaling triggered by R5 human immunodeficiency virus type 1 increases virus replication efficiency in primary T lymphocytes.J Virol. 2005 Jun;79(12):7938-41. doi: 10.1128/JVI.79.12.7938-7941.2005. J Virol. 2005. PMID: 15919952 Free PMC article.
-
Preferential use of CXCR4 by R5X4 human immunodeficiency virus type 1 isolates for infection of primary lymphocytes.J Virol. 2005 Feb;79(3):1480-6. doi: 10.1128/JVI.79.3.1480-1486.2005. J Virol. 2005. PMID: 15650174 Free PMC article.
-
The efficiency of R5 HIV-1 infection is determined by CD4 T-cell surface CCR5 density through G alpha i-protein signalling.AIDS. 2006 Jun 26;20(10):1369-77. doi: 10.1097/01.aids.0000233570.51899.e2. AIDS. 2006. PMID: 16791011
-
The role of viral coreceptors and enhanced macrophage tropism in human immunodeficiency virus type 1 disease progression.Sex Health. 2004;1(1):23-34. doi: 10.1071/sh03006. Sex Health. 2004. PMID: 16335478 Review.
-
HIV-1 Infection in Persons Homozygous for CCR5-Δ32 Allele: The Next Case and the Review.AIDS Rev. 2017 Dec;19(4):219-230. AIDS Rev. 2017. PMID: 28534889 Review.
Cited by
-
Single-Molecule Super-Resolution Imaging of T-Cell Plasma Membrane CD4 Redistribution upon HIV-1 Binding.Viruses. 2021 Jan 19;13(1):142. doi: 10.3390/v13010142. Viruses. 2021. PMID: 33478139 Free PMC article.
-
The apparent interferon resistance of transmitted HIV-1 is possibly a consequence of enhanced replicative fitness.PLoS Pathog. 2022 Nov 18;18(11):e1010973. doi: 10.1371/journal.ppat.1010973. eCollection 2022 Nov. PLoS Pathog. 2022. PMID: 36399512 Free PMC article.
-
HIV controllers exhibit potent CD8 T cell capacity to suppress HIV infection ex vivo and peculiar cytotoxic T lymphocyte activation phenotype.Proc Natl Acad Sci U S A. 2007 Apr 17;104(16):6776-81. doi: 10.1073/pnas.0611244104. Epub 2007 Apr 11. Proc Natl Acad Sci U S A. 2007. PMID: 17428922 Free PMC article.
-
New insights into the mechanisms whereby low molecular weight CCR5 ligands inhibit HIV-1 infection.J Biol Chem. 2011 Feb 18;286(7):4978-90. doi: 10.1074/jbc.M110.168955. Epub 2010 Nov 30. J Biol Chem. 2011. PMID: 21118814 Free PMC article.
-
HIV-1 exploits CCR5 conformational heterogeneity to escape inhibition by chemokines.Proc Natl Acad Sci U S A. 2013 Jun 4;110(23):9475-80. doi: 10.1073/pnas.1222205110. Epub 2013 May 21. Proc Natl Acad Sci U S A. 2013. PMID: 23696662 Free PMC article.
References
-
- Alkhatib, G., M. Locati, P. E. Kennedy, P. M. Murphy, and E. A. Berger. 1997. HIV-1 coreceptor activity of CCR5 and its inhibition by chemokines: independence from G protein signaling and importance of coreceptor downmodulation. Virology 234:340-348. - PubMed
-
- Amara, A., S. L. Gall, O. Schwartz, J. Salamero, M. Montes, P. Loetscher, M. Baggiolini, J. L. Virelizier, and F. Arenzana-Seisdedos. 1997. HIV coreceptor downregulation as antiviral principle: SDF-1alpha-dependent internalization of the chemokine receptor CXCR4 contributes to inhibition of HIV replication. J. Exp. Med. 186:139-146. - PMC - PubMed
-
- Arai, H., and I. F. Charo. 1996. Differential regulation of G-protein-mediated signaling by chemokine receptors. J. Biol. Chem. 271:21814-21819. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

