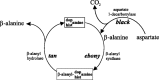
tan and ebony genes regulate a novel pathway for transmitter metabolism at fly photoreceptor terminals
- PMID: 12486147
- PMCID: PMC6758454
- DOI: 10.1523/JNEUROSCI.22-24-10549.2002
tan and ebony genes regulate a novel pathway for transmitter metabolism at fly photoreceptor terminals
Abstract
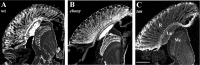
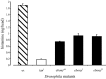
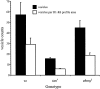
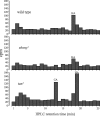
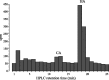
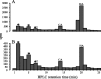
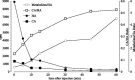
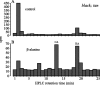
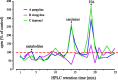
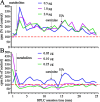
In Drosophila melanogaster, ebony and tan, two cuticle melanizing mutants, regulate the conjugation (ebony) of beta-alanine to dopamine or hydrolysis (tan) of the beta-alanyl conjugate to liberate dopamine. beta-alanine biosynthesis is regulated by black. ebony and tan also exert unexplained reciprocal defects in the electroretinogram, at ON and OFF transients attributable to impaired transmission at photoreceptor synapses, which liberate histamine. Compatible with this impairment, we show that both mutants have reduced histamine contents in the head, as measured by HPLC, and have correspondingly reduced numbers of synaptic vesicles in their photoreceptor terminals. Thus, the histamine phenotype is associated with sites of synaptic transmission at photoreceptors. We demonstrate that when they receive microinjections into the head, wild-type Sarcophaga bullata (in whose larger head such injections are routinely possible) rapidly (<5 sec) convert exogenous [3H]histamine into its beta-alanine conjugate, carcinine, a novel metabolite. Drosophila tan has an increased quantity of [3H]carcinine, the hydrolysis of which is blocked; ebony lacks [3H]carcinine, which it cannot synthesize. Confirming these actions, carcinine rescues the histamine phenotype of ebony, whereas beta-alanine rescues the carcinine phenotype of black;tan double mutants. The equilibrium ratio between [3H]carcinine and [3H]histamine after microinjecting wild-type Sarcophaga favors carcinine hydrolysis, increasing to only 0.5 after 30 min. Our findings help resolve a longstanding conundrum of the involvement of tan and ebony in photoreceptor function. We suggest that reversible synthesis of carcinine occurs in surrounding glia, serving to trap histamine after its release at photoreceptor synapses; subsequent hydrolysis liberates histamine for reuptake.
Figures
Similar articles
-
The role of carcinine in signaling at the Drosophila photoreceptor synapse.PLoS Genet. 2007 Dec;3(12):e206. doi: 10.1371/journal.pgen.0030206. PLoS Genet. 2007. PMID: 18069895 Free PMC article.
-
Alternative tasks of Drosophila tan in neurotransmitter recycling versus cuticle sclerotization disclosed by kinetic properties.J Biol Chem. 2010 Jul 2;285(27):20740-7. doi: 10.1074/jbc.M110.120170. Epub 2010 May 3. J Biol Chem. 2010. PMID: 20439462 Free PMC article.
-
The metabolism of histamine in the Drosophila optic lobe involves an ommatidial pathway: β-alanine recycles through the retina.J Exp Biol. 2012 Apr 15;215(Pt 8):1399-411. doi: 10.1242/jeb.060699. J Exp Biol. 2012. PMID: 22442379 Free PMC article.
-
The dynamics of signaling at the histaminergic photoreceptor synapse of arthropods.Prog Neurobiol. 2007 Jul;82(4):202-27. doi: 10.1016/j.pneurobio.2007.03.006. Epub 2007 Apr 19. Prog Neurobiol. 2007. PMID: 17531368 Review.
-
Pigmentation and behavior: potential association through pleiotropic genes in Drosophila.Genes Genet Syst. 2013;88(3):165-74. doi: 10.1266/ggs.88.165. Genes Genet Syst. 2013. PMID: 24025245 Review.
Cited by
-
Histaminergic Control of Corticostriatal Synaptic Plasticity during Early Postnatal Development.J Neurosci. 2020 Aug 19;40(34):6557-6571. doi: 10.1523/JNEUROSCI.0740-20.2020. Epub 2020 Jul 24. J Neurosci. 2020. PMID: 32709692 Free PMC article.
-
Glial investment of the adult and developing antennal lobe of Drosophila.J Comp Neurol. 2008 Aug 10;509(5):526-50. doi: 10.1002/cne.21762. J Comp Neurol. 2008. PMID: 18537134 Free PMC article.
-
Physiology of Astroglia.Physiol Rev. 2018 Jan 1;98(1):239-389. doi: 10.1152/physrev.00042.2016. Physiol Rev. 2018. PMID: 29351512 Free PMC article. Review.
-
Regulation and modulation of biogenic amine neurotransmission in Drosophila and Caenorhabditis elegans.Front Physiol. 2023 Feb 16;14:970405. doi: 10.3389/fphys.2023.970405. eCollection 2023. Front Physiol. 2023. PMID: 36875033 Free PMC article. Review.
-
Drosophila melanogaster as a genetic model system to study neurotransmitter transporters.Neurochem Int. 2014 Jul;73:71-88. doi: 10.1016/j.neuint.2014.03.015. Epub 2014 Apr 3. Neurochem Int. 2014. PMID: 24704795 Free PMC article. Review.
References
-
- Amara SG, Arriza JL. Neurotransmitter transporters: three distinct gene families. Curr Opin Neurobiol. 1993;3:337–344. - PubMed
-
- Arnould J-M. Biosynthesis and metabolism of histamine in the central nervous system of Carcinus maenas. Arch Int Physiol Biochim. 1985;95:43–55. - PubMed
-
- Arnould J-M. Demonstration of carcinine synthetase, a new enzyme catalysing the metabolism of histamine in the central nervous system of Carcinus maenas. J Neurochem. 1987a;48:1316–1324. - PubMed
-
- Arnould J-M. Beta-alanylation, a means for neutralization of histamine in the central nervous system of Carcinus maenas. Can J Physiol Pharmacol. 1987b;65:1898–1902. - PubMed
-
- Battelle B-A, Hart MK. Histamine metabolism in the visual system of the horseshoe crab Limulus polyphemus. Comp Biochem Physiol. 2002;133:135–142. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

